|
Home
The Team
Project Report
Parts
Modeling
Notebook
Safety
CoLABoration
|
_project report
Summary
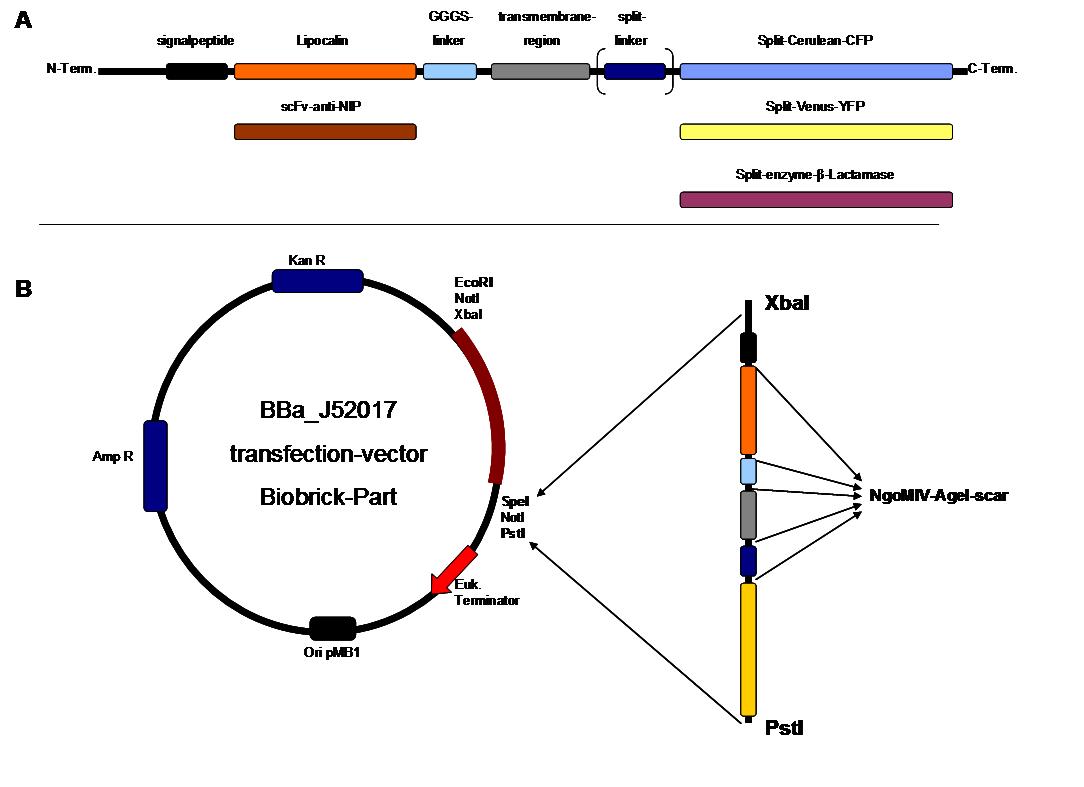
Modular Synthetic Receptor System
In the following we provide a summary of our project and display the highlights of our achievements. For a more detailed view please see the reports of each sub-project.
The general goal of the Freiburg 2008 team is to establish a synthetic transmembrane receptor system comprising the extracellular input devices, the extracellular receiver domain, the transducer to the cytosol and the reporter/executor in the cytosol.
As controllable receptor activation event, we chose spatial control of dimerization, which is a mechanism often used by nature. To achieve spatial control in the nanometer range of proteins we chose DNA origami for exact and complex patterns with several ligands and chemical coupling of one type of ligand to scaffold proteins for simple multimerization.
To facilitate binding, we used a single-chain Fv fragment and a designed anticalin. Both bind haptens which can be conjugated to the DNA-Origami or scaffold proteins. Transduction to the cytosole is mediated by a single transmembrane helix.
As intracellular reporters we employ split proteins. Here, we used the split lactamase from the iGEM2007 Freiburg project as well as the split fluorescent proteins CFP/cerulean and YFP/venus. To provide steric flexibility and reach of our binding and reporting modules we interspersed linkers between each functional domain.
All parts are cloned in in E. coli and feature full BioBrick-compatibility. However, we test our receptors in human or mouse cells/chassis. Thus, to direct our constructs to the cell surface we attached a eukaryotic signal peptide to the N-terminus of each synthetic receptor. In addition to the new synthetic receptors, we also employed an existing T-cell line displaying a TCR fused to a single chain as a test for the spatial control.
Besides designing and cloning parts we spent quite some time on establishing and analyzing the biological experiment. In particular we wanted to test the spatial control via DNA origami using an existing cell line expressing the scFv fused to a T-cell receptor. Here we addressed the following questions:
- Can we improve DNA origami assembly yield by varying the staple oligonucleotide to scaffold DNA ratio?
- Can we find buffer conditions, which mediate cell viability and origami stability?
-Can we downsize a Ca2+ release assay to fit the sample sizes of our precious DNA origami?
- Do our constructs express and then localize in the membrane of eukaryotic cells?
-Can we detect synthetic receptor activation?
To reach our goal within the short given time frame we started several subprojects in parallel. Our sub-projects listed here are defined along these projects. Luckily we did not have to start from total scratch: Daniel Hautzinger, one of our more senior team members, was doing his diploma thesis in the lab of Kristian Müller working with DNA origami while we were also in the lab and Wolfgang Schamel could provide the mentioned T-cell line with a TCR fused to a scFv and a B-cell line directed towards the same antigen.
Subprojects
Modeling
3D-Modeling
DNA-Origami
Cloning Strategy
Cell Culture
Transfection and Synthetic Receptor Activation
Cell Stability, Ca2+ Signaling and DNA-Origami-Binding
Highlights
|
Modeling
The dimerization of the extracellular receptor domains is a important necessity for the functionality of our Modular Synthetic Receptor System. Presenting the system a stimulus in the form of spatial arranged ligands (nitro-iodo-phenol or fluorescein molecules), the extracellular domains dimerize, thus the corresponding intracellular parts such as the split lactamase halves or split fluorescent proteins complement to measureable output. To analyse the theoretical functionality due to dimerization, two receptor dimerization models (one T cell receptor model and one general receptor model) are introduced and discussed and a proper model for the Modular Synthetic Receptor System is constructed. It consists of 10 reaction kinetic equations, 9 ordinary differential equations including 25 variable parameters. Matlab m-files are embedded for further inspection.
|
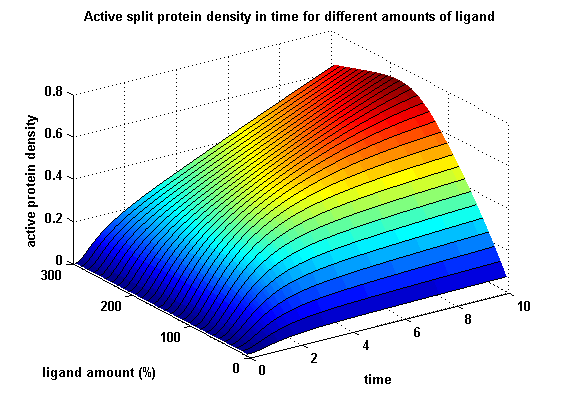 Split protein activity in time dependent on ligand amount
|
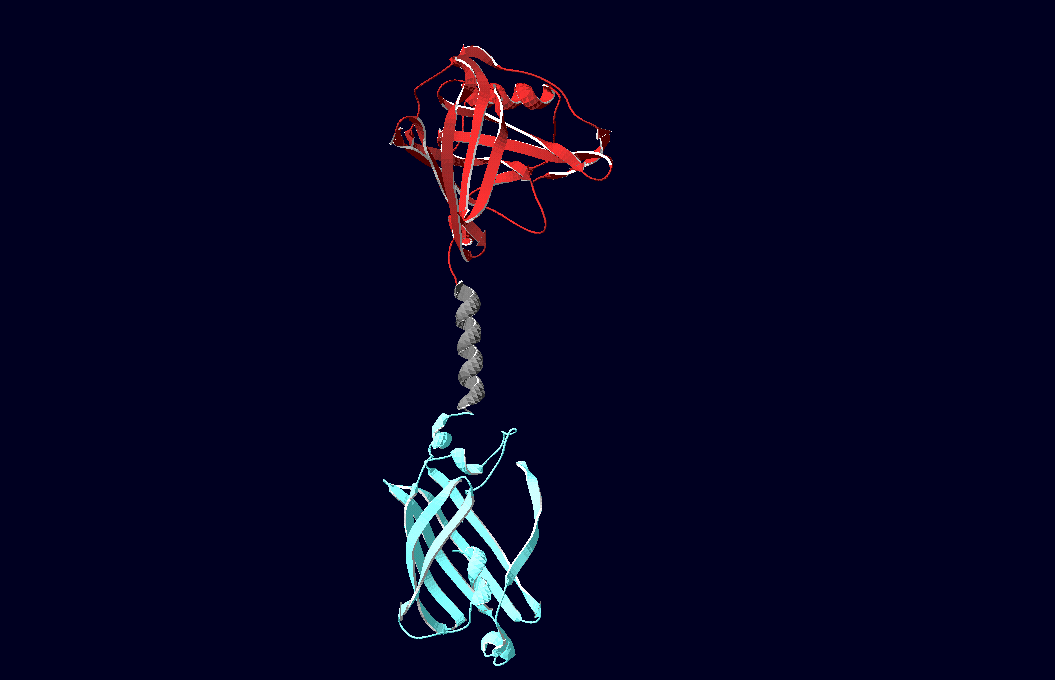 structural model of expression part Bba_K157037 |
3D-Modeling
We have created various three-dimensional models of our constructs using Pymol and SwissPdbViewer.
Pdb-files of the huge DNA-Origami molecule were created as adequate as possible using Nano-Engineer version 1.0 and then edited in Pymol. These Models were used to plan the spatial arrangement and, thus, input-pattern of antigens at a nanometer scale.
|
|
DNA-Origami
The creation of DNA-Origami was one of the first subprojects we engaged in. Structural planing of the input pattern on the Origami-surface and order of the modified oligonucleotides were the first issues, increasing of the Origami-yield and measurement of the molecule´s stability in cell culture media the latter ones.
|
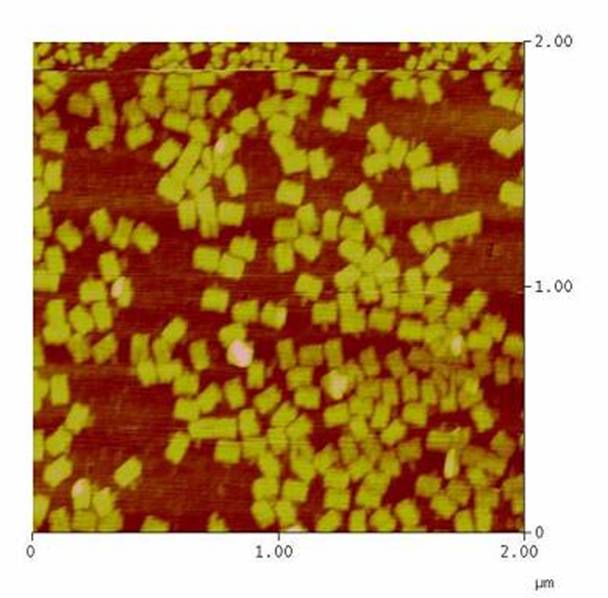 AFM-measurement of DNA-Origami |
|
|
Cloning Strategy
All of our constructs were cloned successfully using our extended pre- and suffix and plasmid "pMA" (part Bba_K157000). The broad combinatorial range given by the modular concept was almost fully exploited, so that we ended up with the submission of 13 basic and 28 composite parts.
Once a construct was completed, it was cloned into the CMV-promoted transfection vector (part Bba_K157040) and used for transfection of 293T-cells.
|
|
Transfection and Synthetic Receptor Activation
Transfection of 293T-cells has also been shown to work for most of our fusion proteins; so far, we could even show that at least our constructs with lipocalin FluA as extracellular domaine are integrated into the membrane.
All transfections were carried out with part Bba_K157040, one of our composite parts consisting of the transfection vector Bba_J52017 and a CMV-promotor. Due to the limited time range we were not able to activate/dimerize any of the various possible "receptor"-pairs yet, but we are hoping to receive a positive result in that concern until the jamboree.
|
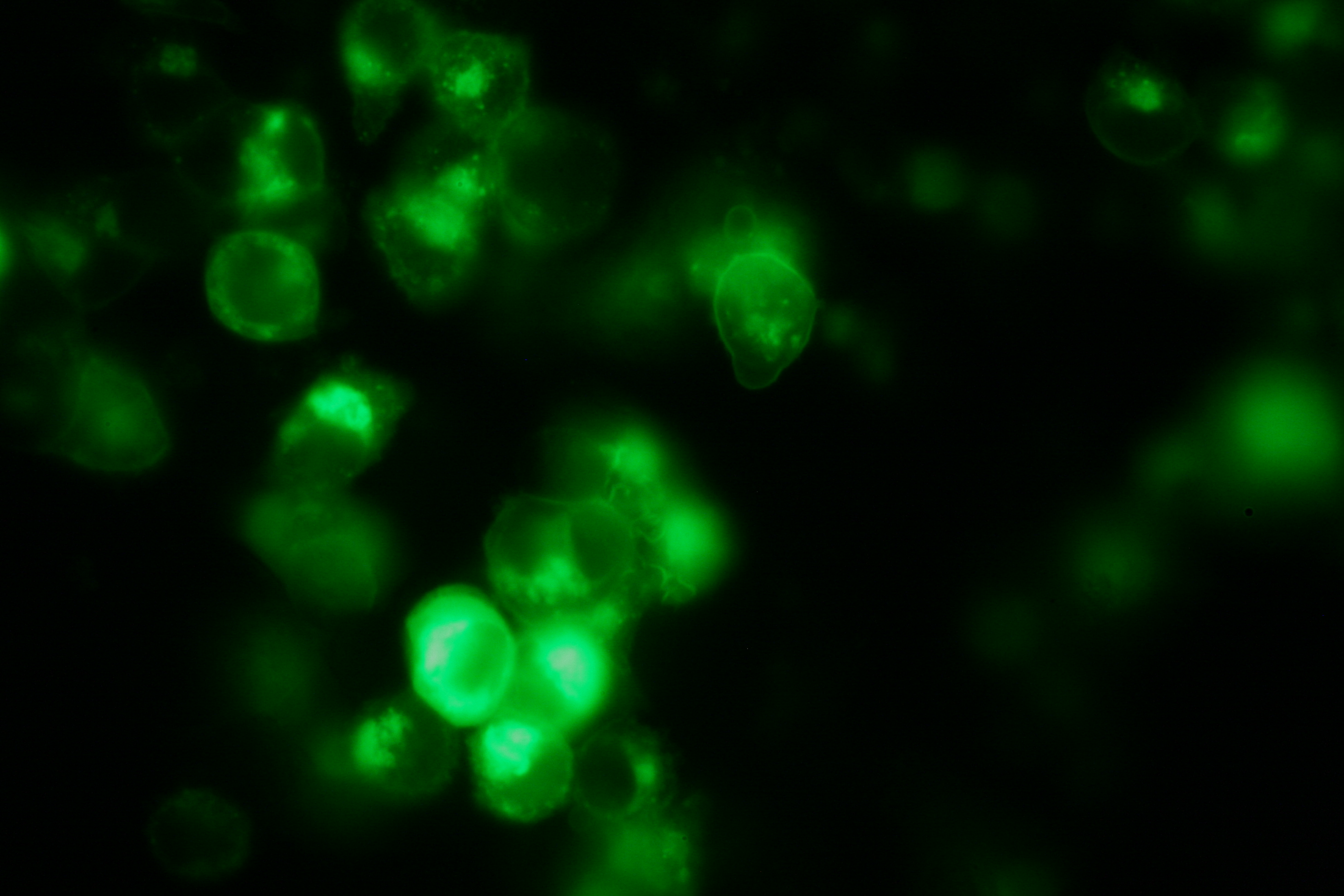 membrane-localization of Part Bba_K157032-YFP |
|
|
Cell Stability, Ca2+ Signaling and DNA-Origami-Binding
Due to the small total amounts of DNA-Origami we could not use FACS to measure calcium influx and, thus, T-cell activation. The alternative we found were "Nano-Slides" for inverse fluorescence microscopy; T-cells were immobilized on the poly-L-Lysine coated slides and stimulated directly under the microscope. This procedure allowed real-time observation of calcium-influx.
|
Literature
Split-fluorophores:
-Chang-Deng Hu, Yurii Chinenov, Tom K. Kerppola: ”Visualization of Interactions among bZIP and Rel Family Proteins in Living Cells Using Bimolecular Fluorescence Complementation”, Molecular Cell, Vol. 9, 789–798, April, 2002
-Chang Deng Hu, Tom K. Kerppola: “Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis”, Nat Biotechnol. 2003 May; 21(5):539-545 (doi:10. 1038/nbt816)
-Tom K. Kerppola: “Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells”, Nat Protoc. 2006;1(3):1278-1286 (doi:10.1038/nprot.2006.201)
-Nagai, T. et al. “A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications” J. Biol. Chem. 276, 29188-29194, 2001
-Roger Y. Tsien et al. „Creating new fluorescent probes for cell biology“, Nature Biotechnology Reviews, Vol. 3, 906-918, 2002
LipocalinFluA:
-Gerald Beste, Frank S. Schmidt, Thomas Stibora and A. Skerra: “Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold“,Proc. Natl. Acad. Sci. USA Vol. 96, pp. 1898–1903, March 1999 Biochemistry
-Ingo P. Korndörfer, Gerald Beste and A. Skerra: “Crystallographic Analysis of an “Anticalin” With Tailored Specificity for Fluorescein Reveals High Structural Plasticity of the Lipocalin Loop Region”, PROTEINS: Structure, Function, and Bioinformatics 53:121–129 (2003)
DNA-Origami:
Paul W. K. Rothemund: Nature 440, 297-302 (16 March 2006)
Antibody B1-8:
-Ana Cumano and Klaus Rajewski: “Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP”, The EMBO Journal vol. 5 no.10 pp. 2459-2468, 1986
-D. Allen, T. Simon, F. Sablitzky, K. Rajewski and A. Cumano: “Antibody engineering for the analysis of affinity maturation of an anti-hapten response”, The EMBO Journal vol. 7 no.7 pp. 1995-2001, 1988
Modeling:
-Martin F. Bachmann, Michael Salzmann, Annette Oxenius and Pamela S. Ohashi: "Formation of TCR dimers/trimers as a crucial step for T cell activation", Eur. J. Immunol., 1998
-Martin F. Bachmann and Pamela S. Ohashi: "The role of T-cell receptor dimerization in T-cell activation", Review Immunology Today, Dezember 1999
-João Sousa and Jorge Carneiro: "A mathematical analysis of TCR serial triggering and down-regulation", Eur. J. Immunol., 2000
TCR activation:
-Susana Minguet, Mahima Swamy, Balbino Alarcón, Immanuel F. Luescher and Wolfgang W.A. Schamel: "Full Activation of the T Cell Receptor requires Both Clustering and Conformational Changes at CD3", Immunity, 2006
|
 "
"