Team:KULeuven/12 August 2008
From 2008.igem.org
m |
(lay-out, typos, extras) |
||
| Line 1: | Line 1: | ||
| + | {{:Team:KULeuven/Tools/Styling}} | ||
{{:Team:KULeuven/Tools/Header}} | {{:Team:KULeuven/Tools/Header}} | ||
| + | |||
| + | {{:Team:KULeuven/Tools/New_Day/Date_Retriever}} | ||
== Lab Work == | == Lab Work == | ||
| Line 5: | Line 8: | ||
=== Wet Lab === | === Wet Lab === | ||
| - | + | The liquid cultures of the colonies with ligation products we prepared yesterday were miniprepped today ([http://partsregistry.org/Part:BBa_J23109 J23109]+[http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_I712074 I712074]+[http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_K145015 K145015]+[http://partsregistry.org/Part:BBa_B0015 B0015], [http://partsregistry.org/Part:BBa_R0062 R0062]+[http://partsregistry.org/Part:BBa_B0032 B0032], [http://partsregistry.org/Part:BBa_R0084 R0084]+[http://partsregistry.org/Part:BBa_J23022 J23022] and [http://partsregistry.org/Part:BBa_C0012 C0012]+[http://partsregistry.org/Part:BBa_B0015 B0015]). After that, we did a PCR to check the ligations. The liquid cultures of parts [http://partsregistry.org/Part:BBa_F1610 F1610], [http://partsregistry.org/Part:BBa_R0040 R0040], [http://partsregistry.org/Part:BBa_J23116 J23116] and [http://partsregistry.org/Part:BBa_P1010 P1010] were also miniprepped. | |
| - | + | ||
| - | + | We made electrocompetent Top10 cells and tested them with pUC. | |
| - | + | ||
| - | + | The cells with parts [http://partsregistry.org/Part:BBa_C0060 C0060]+[http://partsregistry.org/Part:BBa_B0015 B0015], [http://partsregistry.org/Part:BBa_C0040 C0040]+[http://partsregistry.org/Part:BBa_B0015 B0015] and [http://partsregistry.org/Part:BBa_J23100 J23100]+[http://partsregistry.org/Part:BBa_B0032 B0032] we electroporated yesterday gave colonies. They were streaked out and a liquid culture was made. | |
| - | + | ||
| + | The PCR of the T7 polymerase was continued. We tried the same protocol as yesterday, but this time with a longer amplification time, but it failed again. | ||
| + | |||
| + | Some more cutting was done: | ||
| + | * cut with ''Eco''R I and ''Spe'' I: [http://partsregistry.org/Part:BBa_J23116 J23116], [http://partsregistry.org/Part:BBa_K145001 K145001], [http://partsregistry.org/Part:BBa_R0040 R0040], [http://partsregistry.org/Part:BBa_J23109 J23109]+[http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_I712074 I712074]+[http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_R0084 R0084]+[http://partsregistry.org/Part:BBa_J23022 J23022] and [http://partsregistry.org/Part:BBa_R0062 R0062]+[http://partsregistry.org/Part:BBa_B0032 B0032]. | ||
| + | * cut with ''Xba'' I: [http://partsregistry.org/Part:BBa_K145015 K145015]+[http://partsregistry.org/Part:BBa_B0015 B0015], [http://partsregistry.org/Part:BBa_J23109 J23109]+[http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_C0012 C0012]+[http://partsregistry.org/Part:BBa_B0015 B0015], [http://partsregistry.org/Part:BBa_C0062 C0062]+[http://partsregistry.org/Part:BBa_B0015 B0015] and [http://partsregistry.org/Part:BBa_E0022 E0022]+[http://partsregistry.org/Part:BBa_B0015 B0015]. | ||
=== Dry Lab === | === Dry Lab === | ||
| - | == Modeling == | + | ==== Modeling ==== |
| - | Great news today: issues from yesterday seem to be solved! Some more inaccuracies were found, but in a | + | Great news today: issues from yesterday seem to be solved! Some more inaccuracies were found, but in a mysterious way, they seem to cancel out each other in the new model. Also, we included the latest version of the memory (hopefully this time the final version) in the full model without problems. |
| - | In the afternoon the good spirit was brought back to zero by the discovery of some | + | In the afternoon the good spirit was brought back to zero by the discovery of some inconsistencies in the modeling of the T7 promotor. New research had to be done and this resulted in depressing simulation results: a background noise level which completely overwhelmed our system. |
A good sleep will bring more insight and better results! | A good sleep will bring more insight and better results! | ||
| - | == Wiki == | + | ==== Wiki ==== |
Tabs plugin inserted after much confusion, code reformatted. Picture gallery setup. For the rest: 1011001011 and 101100001101, oh and 011001... | Tabs plugin inserted after much confusion, code reformatted. Picture gallery setup. For the rest: 1011001011 and 101100001101, oh and 011001... | ||
| Line 28: | Line 36: | ||
== Remarks == | == Remarks == | ||
[[Image:TGS.PNG|center]] | [[Image:TGS.PNG|center]] | ||
| - | |||
| - | |||
Latest revision as of 12:29, 11 October 2008
| << return to notebook | return to homepage >> | ||
| < previous friday | ← yesterday | tomorrow → | next monday > |
Contents |
Lab Work
Wet Lab
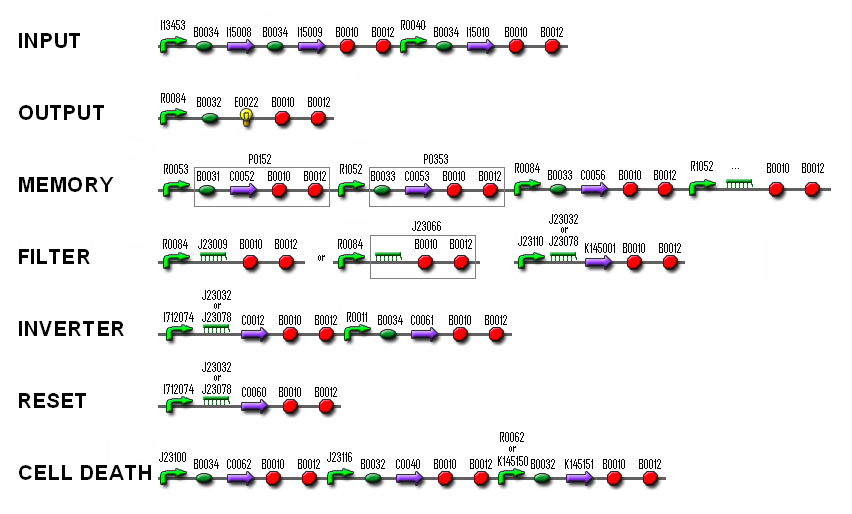
The liquid cultures of the colonies with ligation products we prepared yesterday were miniprepped today (J23109+J23032, I712074+J23032, K145015+B0015, R0062+B0032, R0084+J23022 and C0012+B0015). After that, we did a PCR to check the ligations. The liquid cultures of parts F1610, R0040, J23116 and P1010 were also miniprepped.
We made electrocompetent Top10 cells and tested them with pUC.
The cells with parts C0060+B0015, C0040+B0015 and J23100+B0032 we electroporated yesterday gave colonies. They were streaked out and a liquid culture was made.
The PCR of the T7 polymerase was continued. We tried the same protocol as yesterday, but this time with a longer amplification time, but it failed again.
Some more cutting was done:
- cut with EcoR I and Spe I: J23116, K145001, R0040, J23109+J23032, I712074+J23032, R0084+J23022 and R0062+B0032.
- cut with Xba I: K145015+B0015, J23109+J23032, C0012+B0015, C0062+B0015 and E0022+B0015.
Dry Lab
Modeling
Great news today: issues from yesterday seem to be solved! Some more inaccuracies were found, but in a mysterious way, they seem to cancel out each other in the new model. Also, we included the latest version of the memory (hopefully this time the final version) in the full model without problems.
In the afternoon the good spirit was brought back to zero by the discovery of some inconsistencies in the modeling of the T7 promotor. New research had to be done and this resulted in depressing simulation results: a background noise level which completely overwhelmed our system.
A good sleep will bring more insight and better results!
Wiki
Tabs plugin inserted after much confusion, code reformatted. Picture gallery setup. For the rest: 1011001011 and 101100001101, oh and 011001...
 "
"