Team:KULeuven/18 August 2008
From 2008.igem.org
m |
(lay-out) |
||
| Line 1: | Line 1: | ||
| + | {{:Team:KULeuven/Tools/Styling}} | ||
{{:Team:KULeuven/Tools/Header}} | {{:Team:KULeuven/Tools/Header}} | ||
| + | |||
| + | {{:Team:KULeuven/Tools/New_Day/Date_Retriever}} | ||
== Lab Work == | == Lab Work == | ||
=== Wet Lab === | === Wet Lab === | ||
| - | + | [[image:gel-18-8a.jpg|right|thumb|340px|'''Gel 1''': PCR product of K145151 and K145013]] | |
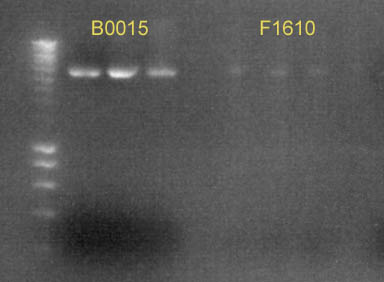
| - | + | [[image:gel-18-8b.jpg|right|thumb|340px|'''Gel 2''': Digest of B0015 and F1610 with ''Xba'' I]] | |
| - | + | ||
| - | + | We did some PCRs with ''pfx'' polymerase to prepare the following (new) BioBricks: [http://partsregistry.org/Part:BBa_K145014 K145014] (T7 polymerase with UmuD), [http://partsregistry.org/Part:BBa_K145013 K145013] (antisense LuxI) and [http://partsregistry.org/Part:BBa_K145151 K145151] (ccdB). The templates and primers used were, respectively, PCR of step 1 with 1077/1079, BBa_F1610 with 1100/1101, BBa_P1010 with 1127/1128. Only the PCR of ccdB succeeded. (see '''Gel 1'''). | |
| + | |||
| + | We made a few digests: [http://partsregistry.org/Part:BBa_F1610 F1610] and [http://partsregistry.org/Part:BBa_B0015 B0015] were cut with ''Eco''RI and ''Xba''I (see '''Gel 2'''), and [http://partsregistry.org/Part:BBa_J23100 J23100]+[http://partsregistry.org/Part:BBa_B0032 B0032] and [http://partsregistry.org/Part:BBa_R0062 R0062]+[http://partsregistry.org/Part:BBa_B0032 B0032] were cut with ''Eco''RI and ''Spe''I. | ||
| + | |||
| + | We electroporated the following ligated parts: [http://partsregistry.org/Part:BBa_C0040 C0040]+[http://partsregistry.org/Part:BBa_B0015 B0015] , [http://partsregistry.org/Part:BBa_K145015 K145015]+[http://partsregistry.org/Part:BBa_B0015 B0015], [http://partsregistry.org/Part:BBa_C0012 C0012]+[http://partsregistry.org/Part:BBa_B0015 B0015] and [http://partsregistry.org/Part:BBa_C0060 C0060]+[http://partsregistry.org/Part:BBa_B0015 B0015]. | ||
| + | |||
| + | We continued ligating: [http://partsregistry.org/Part:BBa_J23116 J23116]+[http://partsregistry.org/Part:BBa_B0032 B0032] , [http://partsregistry.org/Part:BBa_K145001 K145001]+[http://partsregistry.org/Part:pSB1A2 pSB1A2], [http://partsregistry.org/Part:BBa_R0040 R0040]+[http://partsregistry.org/Part:BBa_B0032 B0032], [http://partsregistry.org/Part:BBa_R0040 R0040]+[http://partsregistry.org/Part:BBa_J23022 J23022], [http://partsregistry.org/Part:BBa_R0084 R0084]+[http://partsregistry.org/Part:BBa_B0032 B0032], [http://partsregistry.org/Part:BBa_K145001 K145001]+[http://partsregistry.org/Part:BBa_B0015 B0015] and ([http://partsregistry.org/Part:BBa_R0084 R0084]+[http://partsregistry.org/Part:BBa_J23022 J23022])+([http://partsregistry.org/Part:BBa_J23109 J23109]+[http://partsregistry.org/Part:BBa_J23032 J23032]). This time, B0032 was first dephosphorylated. | ||
=== Dry Lab === | === Dry Lab === | ||
| Line 23: | Line 32: | ||
== Remarks == | == Remarks == | ||
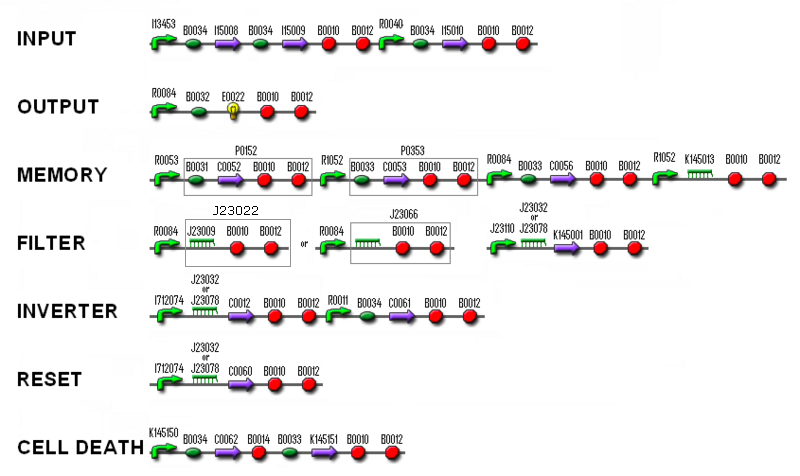
[[Image:TGS2.PNG | center]] | [[Image:TGS2.PNG | center]] | ||
| - | |||
| - | |||
Latest revision as of 16:27, 11 October 2008
| << return to notebook | return to homepage >> | ||
| < previous friday | ← yesterday | tomorrow → | next monday > |
Contents |
Lab Work
Wet Lab
We did some PCRs with pfx polymerase to prepare the following (new) BioBricks: K145014 (T7 polymerase with UmuD), K145013 (antisense LuxI) and K145151 (ccdB). The templates and primers used were, respectively, PCR of step 1 with 1077/1079, BBa_F1610 with 1100/1101, BBa_P1010 with 1127/1128. Only the PCR of ccdB succeeded. (see Gel 1).
We made a few digests: F1610 and B0015 were cut with EcoRI and XbaI (see Gel 2), and J23100+B0032 and R0062+B0032 were cut with EcoRI and SpeI.
We electroporated the following ligated parts: C0040+B0015 , K145015+B0015, C0012+B0015 and C0060+B0015.
We continued ligating: J23116+B0032 , K145001+pSB1A2, R0040+B0032, R0040+J23022, R0084+B0032, K145001+B0015 and (R0084+J23022)+(J23109+J23032). This time, B0032 was first dephosphorylated.
Dry Lab
Modeling
Association and dissociation rates have been reinvestigated (HSL with LuxR), wrong values have been removed, LuxR autoactivation has been further incorporated; we've also included diffusion of HSL.
Wiki
Small updates for the wiki: adjusting pictograms, home page, ...
Started defining a global css, intermediate result here: Team:KULeuven/Zandbak/Global
 "
"