Team:KULeuven/6 August 2008
From 2008.igem.org
m |
(lay-out) |
||
| Line 1: | Line 1: | ||
{{:Team:KULeuven/Tools/Header}} | {{:Team:KULeuven/Tools/Header}} | ||
| + | {{:Team:KULeuven/Tools/Styling}} | ||
| + | |||
| + | {{:Team:KULeuven/Tools/New_Day/Date_Retriever}} | ||
== Lab Work == | == Lab Work == | ||
=== Wet Lab === | === Wet Lab === | ||
| - | + | The electrocompetent cells were tested with pUC and we had a lot of colonies so these are working great. Ligation products were transformed to these electrocompetent cells ([http://partsregistry.org/Part:BBa_E0022 E0022]+[http://partsregistry.org/Part:BBa_B0015 B0015], [http://partsregistry.org/Part:BBa_J23109 J23109]+[http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_I712074 I712074]+[http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_K145015 K145015]+[http://partsregistry.org/Part:BBa_B0015 B0015], [http://partsregistry.org/Part:BBa_K145015 K145015]+[http://partsregistry.org/Part:BBa_pSB1A2 pSB1A2], [http://partsregistry.org/Part:BBa_C0062 C0062]+[http://partsregistry.org/Part:BBa_B0015 B0015] and pUC as a control). | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
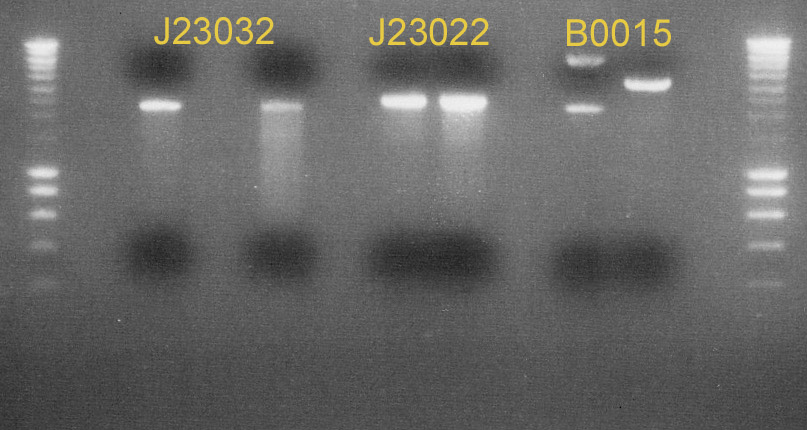
| - | + | We miniprepped the colonies of the ligations that succeeded so fat. We also made digest of [http://partsregistry.org/Part:BBa_J23032 J23032], [http://partsregistry.org/Part:BBa_F1610 F1610], [http://partsregistry.org/Part:BBa_J23022 J23022] and [http://partsregistry.org/Part:BBa_B0015 B0015]. A picture of the result can be found below. | |
| + | The three punched parts of the memory gave a few colonies. One of them did not give any colonies at all. The colonies we had were plated on new plates. | ||
| + | |||
| + | [[image:gel-06-8.jpg|center|thumb|700px|Picture of two gels containing the digests <br> '''Gel 1''' (left) J23032, J23022 and B0015, '''Gel 2''' (right) F1610]] | ||
| + | |||
| + | === Dry Lab === | ||
| + | ==== General ==== | ||
Check possibility of hybrid promoter to connect memory and timer (LuxI). This is to replace the antisense LuxI. Promotor would look something like this: <br> | Check possibility of hybrid promoter to connect memory and timer (LuxI). This is to replace the antisense LuxI. Promotor would look something like this: <br> | ||
Operator<sub>HSL_LuxR</sub> '''---''' -35 box '''---''' Operator<sub>CII P22</sub> '''---''' -10 box | Operator<sub>HSL_LuxR</sub> '''---''' -35 box '''---''' Operator<sub>CII P22</sub> '''---''' -10 box | ||
| - | |||
| - | |||
Hybrid promoter has been made! [http://partsregistry.org/Part:BBa_K145150 Hooray for BBa_K145150!] It should be activated by HSL-LuxR and repressed by P22 C2 (which is produced when the memory is OFF, hereby repressing LuxI synthesis) Check tomorrow how to best obtain the sequence. | Hybrid promoter has been made! [http://partsregistry.org/Part:BBa_K145150 Hooray for BBa_K145150!] It should be activated by HSL-LuxR and repressed by P22 C2 (which is produced when the memory is OFF, hereby repressing LuxI synthesis) Check tomorrow how to best obtain the sequence. | ||
| - | |||
'''Literature''' <br> | '''Literature''' <br> | ||
| Line 27: | Line 29: | ||
PS: P22 C2 is homologous to lambda CI while P22 C1 is homologous to lambda CII | PS: P22 C2 is homologous to lambda CI while P22 C1 is homologous to lambda CII | ||
| - | == Modeling == | + | ==== Modeling ==== |
| - | The antisense part has been remodeled to more accurately show its leakiness. This however appears to be | + | The antisense part has been remodeled to more accurately show its leakiness. This however appears not to be a problem at all. Plus we can always replace it with the hybrid promoter. |
| - | Started work on the sensitivity analyses. So far so good as it would appear. | + | Started work on the sensitivity analyses. So far so good, as it would appear. |
Further work on the memory because we got some new parameters. The switching has become somewhat trickier now. | Further work on the memory because we got some new parameters. The switching has become somewhat trickier now. | ||
| - | == Wiki == | + | New modeling of the antisense LuxI and its target LuxI mRNA shows that this leaky repression is not a problem for the system. Still looking at the composite promoter though. |
| + | |||
| + | ==== Wiki ==== | ||
Dr. Coli is everywhere, even at the URL. | Dr. Coli is everywhere, even at the URL. | ||
| Line 41: | Line 45: | ||
== Remarks == | == Remarks == | ||
| - | Discovery of the day. Two people beat us to the punch: [http://www.diagnosticinformationsystem.com/bios.html 1] and [http://www.sscofcny.com/doctors/coli.htm 2] | + | * Discovery of the day. Two people beat us to the punch: [http://www.diagnosticinformationsystem.com/bios.html 1] and [http://www.sscofcny.com/doctors/coli.htm 2] |
| - | + | * Sindsdien hangt uw haar slap - Maarten | |
| - | Sindsdien hangt uw haar slap -Maarten | + | |
| - | + | ||
| - | + | ||
Revision as of 15:28, 10 October 2008
| << return to notebook | return to homepage >> | ||
| < previous friday | ← yesterday | tomorrow → | next monday > |
Contents |
Lab Work
Wet Lab
The electrocompetent cells were tested with pUC and we had a lot of colonies so these are working great. Ligation products were transformed to these electrocompetent cells (E0022+B0015, J23109+J23032, I712074+J23032, K145015+B0015, K145015+pSB1A2, C0062+B0015 and pUC as a control).
We miniprepped the colonies of the ligations that succeeded so fat. We also made digest of J23032, F1610, J23022 and B0015. A picture of the result can be found below.
The three punched parts of the memory gave a few colonies. One of them did not give any colonies at all. The colonies we had were plated on new plates.
Dry Lab
General
Check possibility of hybrid promoter to connect memory and timer (LuxI). This is to replace the antisense LuxI. Promotor would look something like this:
OperatorHSL_LuxR --- -35 box --- OperatorCII P22 --- -10 box
Hybrid promoter has been made! Hooray for BBa_K145150! It should be activated by HSL-LuxR and repressed by P22 C2 (which is produced when the memory is OFF, hereby repressing LuxI synthesis) Check tomorrow how to best obtain the sequence.
Literature
Lux regulatory region (pLux)
PS: P22 C2 is homologous to lambda CI while P22 C1 is homologous to lambda CII
Modeling
The antisense part has been remodeled to more accurately show its leakiness. This however appears not to be a problem at all. Plus we can always replace it with the hybrid promoter.
Started work on the sensitivity analyses. So far so good, as it would appear.
Further work on the memory because we got some new parameters. The switching has become somewhat trickier now.
New modeling of the antisense LuxI and its target LuxI mRNA shows that this leaky repression is not a problem for the system. Still looking at the composite promoter though.
Wiki
Dr. Coli is everywhere, even at the URL.
Remarks
- Sindsdien hangt uw haar slap - Maarten
 "
"