Team:KULeuven/Data/Other Components
From 2008.igem.org
Contents |
Introduction
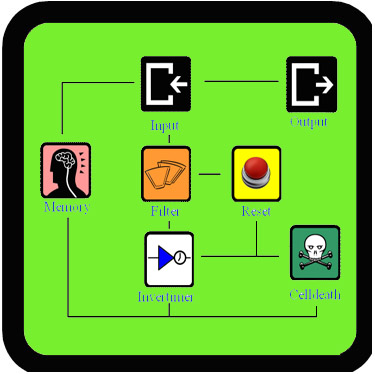
As Dr.Coli is a very extensive project, there was not enough time to test all the new parts that we created. This page contains a summary of how our components can be tested (the construct that have to be made and the actual testing protocol). It will also give an overview of the results we had so far. We worked as much parallel as possible, building different parts and subsystems at the same time. This way we could still have some results, even when some of the devices didn't succeed. Some of the constructs are already built, but the actual testing and finishing of the parts will have to be done be next year's teams, our advisors or some other students.
Filter
Parts Registry:K145215 and Parts Registry:K145216
Construct & protocol
Our filter is a composite part that is designed to filter out background noise of input. It can do this by a feedforward mechanism that uses an AND-gate. It works like this: if there is an input signal, the filter will first produce a ribokey. This key will then unlock a ribolock and this will lead to the expression of T7 RNA polymerase. Both this key and T7 RNA polymerase are the input of the AND-gate (which consists of a T7 promoter and ribolock) and both are necessary to get output from this AND-gate. So, when the input signal is very short, the ribokey will already be degraded by the time that T7 RNA polymerase is produced. In that case the AND-gate will not give an output. But when the input signal is longer, ribokey will still be available when T7 RNA polymerase is produced and therefore the AND-gate will produce an output. This way the filter can distinguish short signals (noise) from long signals (relevant signals).
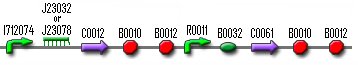
So if we want to test the filter, we need an input system (which is a constitutively produced TetR that will switch off the filter until aTc is added), the filter and a GFP connected to the AND-gate:
| + | + | |||
| Input | Filter | AND-gate with GFP |
Once this construct is made, testing the system becomes pretty straightforward. You would have to prepare multiple liquid cultures of the cells containing a plasmid with the above construct. Ten you can add aTc and remove it again after various time intervals (e.g. by transferring the cells to a fresh medium with no aTc). This will establish input signals of different lengths and you can detect the effect of each input signal by measuring the fluorescence of GFP (e.g. with FACS). You should detect no fluorescence when you gave a short input signal and you should be able to detect fluorescence when the input signal was longer. If you prepare various samples with various input lengths, you can make a plot of the fluorescence as a function of the input duration. This way you should be able to determine the threshold of the filter (which signals does the filter filter out, and which ones does he let through?).
Results
We did a lot of PCRs, digesting and ligating to prepare the construct. The input and the ANG-gate with GFP were properly constructed, as was the first half of the filter (R0040+J23066). But we had some problems with the second part of the filter. In the beginning the PCRs to prepare T7 polymerase failed over and over again. When it finally did succeed, we couldn't manage to insert this T7 RNA polymerase gene into the rest of the filter. So this test module was only partially built.
InverTimer, Reset, Cell Death
Construct & protocol
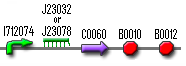
The InverTimer, Reset and Cell Death are essential subsystems that allow controlled cell death when Dr.Coli is no longer needed to cure the patient. This means that these parts are very tightly connected to each other and testing them separately would be very challenging. In order to test these systems, we would actually have to build the full model and then see how Dr.Coli reacts on different input signals (see full model). This could never be done within three months, but we started to build these parts anyway, so that someone else can finish it. These are the constructs:
| InverTimer: | |
| Reset: | |
| Cell Death: |
Results
In order to make the Cell Death construct, we had to make a hybrid promoter (K145150) and the ccdB gene (K145151). This succeeded, but we couldn’t finish the whole construct.
The InverTimer and the Reset were completely built using the standard assembly method. But sequencing showed that we made a little mistake: we built in the wrong promoter. We then tried to undo this mistake by designing primers that can change the promoter to the correct one. This PCR succeeded, and so we digested the PCR products with EcoRI and XbaI and ligated it into pSB1A2. These plasmids were then electroporated into Top10, but we never got any colonies (we tried it a couple of times). We sent the other parts to the Registry anyway (K245300 and K145302); maybe someone else can use them in another project.
 "
"