Team:Montreal
From 2008.igem.org
Horiavulpe (Talk | contribs) |
|||
| Line 1: | Line 1: | ||
{| align="center" | {| align="center" | ||
| - | |align="center" |[[Image:mcgill front2.jpg|850 px | + | |align="center" |[[Image:mcgill front2.jpg|850 px]] |
|}<br> | |}<br> | ||
{|style="font color="#ffffff"; background-color:#cd0000; cellpadding="3" cellspacing="5" border="2" bordercolor="#cd0000"border-spacing:6px; text-align:center" width="960px" | {|style="font color="#ffffff"; background-color:#cd0000; cellpadding="3" cellspacing="5" border="2" bordercolor="#cd0000"border-spacing:6px; text-align:center" width="960px" | ||
| Line 19: | Line 19: | ||
===Background=== | ===Background=== | ||
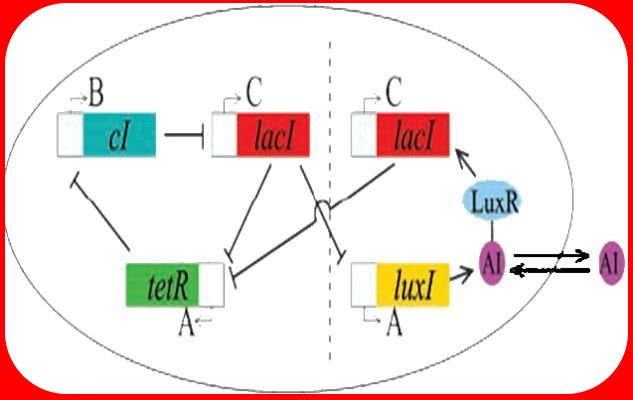
| - | [[Image: | + | [[Image:Osc-rep.jpg||thumb|left|300px | A sinusoidal wave demonstrates a circadian rhythm]]Although typically used to describe physical phenomena, oscillations are also observed in a range of biological processes such as the circadian rhythm, neuronal communication and nephron function. [http://www.aph.caltech.edu/people/elowitz_m.html Elowitz] and Leibler ([http://www.aph.caltech.edu/people/Repressilator.pdf 2000]) postulated that a genetic system called the Repressilator, composed of three genes (λcI, TetR, LacI) tied in negative feedback loops, would generate oscillations in protein expression within ''Escherichia coli''. However, the Repressilator does not allow for synchronization of oscillations in a population of cells. We attempt to bring oscillating cells into phase with eachother by coupling via the "autoinducer" molecule AI. Our 2-gene construct may also be used in other systems, making it a system of choice in a highly modular genetic engineering framework. |
<br><br><br> | <br><br><br> | ||
<br> | <br> | ||
Revision as of 04:00, 6 August 2008
|
| Home | The Team | The Project | Parts | Notebook | Modeling | Links | Sponsors |
|---|
Project Overview: Elucidating an Experimentally Viable Repressilator
Background
Although typically used to describe physical phenomena, oscillations are also observed in a range of biological processes such as the circadian rhythm, neuronal communication and nephron function. Elowitz and Leibler (2000) postulated that a genetic system called the Repressilator, composed of three genes (λcI, TetR, LacI) tied in negative feedback loops, would generate oscillations in protein expression within Escherichia coli. However, the Repressilator does not allow for synchronization of oscillations in a population of cells. We attempt to bring oscillating cells into phase with eachother by coupling via the "autoinducer" molecule AI. Our 2-gene construct may also be used in other systems, making it a system of choice in a highly modular genetic engineering framework.
Relevance and inspiration
While it may be presumptuous to call Elowitz's Repressilator the proverbial 'Holy Grail' of modern bioengineering, parallels can be drawn between these two that tempt the leap to association. The Repressilator, often theorized about but never reproduced in a laboratory, is a crucial stepping stone towards a true understanding of synthetic oscillatory systems and the road towards creating a synthetic system that emulate the natural. Innumerable past, present and future bioengineering projects in iGEM and the real scientific community focus and revolve around this concept and its applications. By exploring the fundamental theory through mathematical models and empirical observation, we hope to understand the nature of these systems and develop future synthetic applications. •
 "
"