Team:UC Berkeley/LayeredAssembly
From 2008.igem.org
Dirkvandepol (Talk | contribs) (→Project Motivation) |
m (→Entry Layer) |
||
| (61 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
{{UCBmain}} | {{UCBmain}} | ||
<div style="text-align: center;"><font size="6">'''Project Motivation'''</font></div><br> | <div style="text-align: center;"><font size="6">'''Project Motivation'''</font></div><br> | ||
| Line 4: | Line 5: | ||
=='''Project Motivation'''== | =='''Project Motivation'''== | ||
| - | Imagine a world with no mini-preps. A world where expensive cloning reagents were unnecessary. A world where DNA extraction and purification could be accomplished in a single | + | Imagine a world with no mini-preps. A world where expensive cloning reagents were unnecessary. A world where DNA and protein extraction and purification could be accomplished in a single microcentrifuge tube. We did, and so we created Clonebots, an automated approach to synthetic biology. |
| - | + | Clonebots seeks to simplify and automate common laboratory procedures to protocols involving only liquid handling steps that can be readily performed by robots. We will accomplish this by genetically encoding many of the required steps in ''E. coli'', thereby allowing the cells to perform the required protocols ''in vivo''. We worked on a wide variety of devices for these projects, and the details about our devices are described throughout this wiki. Most of our devices are associated with biologically encoding the reactions needed for standard assembly. | |
=='''Layered Assembly'''== | =='''Layered Assembly'''== | ||
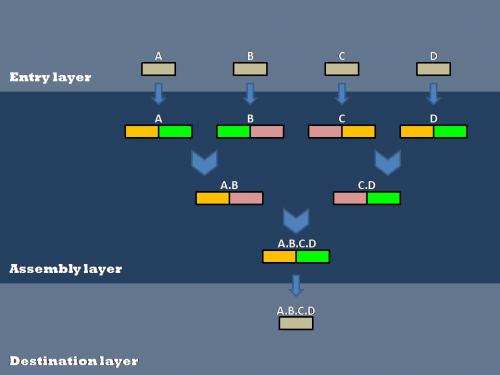
| - | Our efforts to | + | Our efforts to genetically encode standard assembly protocols centered around the layered assembly scheme used by the Anderson Lab at UC Berkeley. Layered assembly is a straight-forward and robust method for combining two or more basic parts into a destination vector. It involves three basic layers of vectors: Entry, Assembly and Destination. Parts are passed between the layers using Gateway reactions (normal arrows) and are assembled within the Assembly layer using 2ab reactions (Chevron arrows), a variation on Biobrick standard assembly. Entry and Assembly vectors are standardized for ease of use, while the Destination vectors can be tailored to a specific experiment or assay. |
[[Image:layered Assembly.jpg|center|frame|350px|A schematic representation of Layered Assembly]] | [[Image:layered Assembly.jpg|center|frame|350px|A schematic representation of Layered Assembly]] | ||
| Line 16: | Line 17: | ||
==='''Entry Layer'''=== | ==='''Entry Layer'''=== | ||
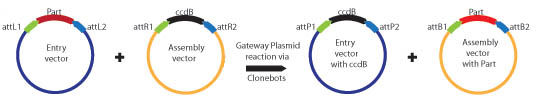
| - | Biobrick parts are created in an entry vector. The Entry vector contains a Spectinomycin antibiotic resistance marker and EcoRI, BglII and BamHI | + | Biobrick parts are created in an entry vector. The Entry vector contains a Spectinomycin antibiotic resistance marker and Biobrick restriction sites (EcoRI, XbaI, and SpeI for the BBa standard; EcoRI, BglII, and BamHI for the BBb1 standard). The restriction sites are flanked by attR1 and attR2 recombination sites. Since the entry plasmid contains attR recombination sites, the basic part can easily be transferred into one of six different assembly vectors using the Gateway cloning scheme: |
| - | [[Image: | + | [[Image: entry plasmid.jpg]] |
| - | + | ||
| - | + | ||
==='''Assembly Layer'''=== | ==='''Assembly Layer'''=== | ||
| - | The | + | The Assembly layer is used to combine two or more basic parts in a specified order to create a composite part. |
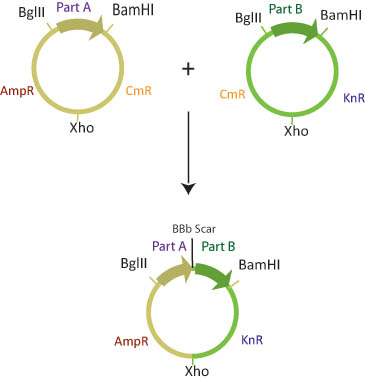
| - | There are six assembly plasmids which contain two of three different antibiotic resistance markers (kanamycin, | + | There are six assembly plasmids which contain two of three different antibiotic resistance markers (kanamycin, ampicillin and Chloramphenicol) separated by a XhoI restriction site. The basic parts are flanked by BglII and BamHI restriction sites. |
The choice of antibiotic resistance markers in the assembly plasmids is predetermined such that once the two plasmids recombine, the new plasmid will have a combination of markers from the two assembly vectors. Although there are four possible products of this reaction, only one will have both of the correct antibiotic resistance markers. | The choice of antibiotic resistance markers in the assembly plasmids is predetermined such that once the two plasmids recombine, the new plasmid will have a combination of markers from the two assembly vectors. Although there are four possible products of this reaction, only one will have both of the correct antibiotic resistance markers. | ||
| - | The Clotho program developed by the UC Berkeley Comp team generates an assembly tree that minimizes the number of reactions required to make a composite part. | + | The [[Team:UC_Berkeley_Tools/Project/Downloads|Clotho]] program developed by the UC Berkeley Comp team generates an assembly tree that minimizes the number of reactions required to make a composite part. |
| - | + | The Assembly plasmids are transformed into cells that are engineered to methylate either BamHI or BglII restriction sites. Part A is transformed into a "lefty" cell that is methylated on BglII restriction sites while Part B is transformed into a "righty" cell that is methylated on BamHI restriction sites. | |
| - | + | In a single microcentrifuge tube, lefty and righty plasmid DNA is combined, along with BamHI, BglII, XhoI restriction enzymes and ligase. Since restriction enzymes will not cut methylated DNA, the BglII restriction site in the lefty cell and the BamHI restriction site in the righty cell are blocked from digestion. The BamHI site in lefty and the BglII site in righty, and the XhoI sites in both plasmids will be cut. Ligase will join the XhoI sites and the BglII/BamHI complimentary sticky ends to create a composite part with a different combination of antibiotic resistance genes than either parent plasmid. | |
| - | + | ||
| - | In a single | + | |
[[Image:cdb2ab.jpg]] | [[Image:cdb2ab.jpg]] | ||
| Line 42: | Line 39: | ||
The assembly reaction can be repeated to combine a number of basic parts in a desired order. | The assembly reaction can be repeated to combine a number of basic parts in a desired order. | ||
| - | Once the composite part is complete, it is transferred to a destination plasmid. | + | Once the composite part is complete, it is transferred to a destination plasmid. |
==='''Destination Layer'''=== | ==='''Destination Layer'''=== | ||
| - | Completed composite parts are transferred from the assembly layer to the destination layer using the Gateway cloning scheme | + | Completed composite parts are transferred from the assembly layer to the destination layer using the Gateway cloning scheme. The destination plasmid is tailored to a specific experiment or assay. This allows a single composite part to be transferred to several different destination plasmids for further experimentation or characterization. |
| - | + | ||
| - | + | ||
=='''Issues with Current Methods'''== | =='''Issues with Current Methods'''== | ||
| + | # Current protocols for standard assembly work pretty well, but assembly of basic parts remains the time and cost-limiting aspect of synthetic biology research. Clonebots will help us simplify standard assembly procedures making them more amenable to large-scale automation. | ||
| + | # Gateway reactions, used for transferring basic parts into the assembly plasmids and transferring composite parts into destination plasmids, are expensive ($8.65/reaction). | ||
| + | # Mini-preps are time-consuming and expensive. | ||
| + | # People are more prone to make mistakes, i.e. switch tubes, use the wrong enzymes, etc. than robots. | ||
| - | + | =='''Clonebots Solutions'''== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==''' | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | # Engineer cells to express their own integrase (Int), excisionase (Xis), and integration host factor (IHF) enzymes to perform ''in vivo'' Gateway reactions. | |
| + | # Eliminate mini-prep steps from cloning protocols by using our lysis device and ''in vivo'' assembly. | ||
| + | # Engineer cells and plasmids to express their own BamHI, BglII, Cre, and ligase enzymes so that assembly reactions in cell lysate and ''in vivo'' are possible. | ||
| + | # Develop protocols that involve only liquid handling steps, so that cloning can be automated, requiring less money and labor. | ||
| - | + | <html> | |
| + | <a href="https://2008.igem.org/Team:UC_Berkeley/GatewayOverview" class="titleIcon"> | ||
| + | <img align=right src="https://static.igem.org/mediawiki/2008/9/9a/GreyNext.png"> | ||
| + | </a> | ||
| + | </html> | ||
Latest revision as of 06:45, 30 October 2008
Project Motivation
Imagine a world with no mini-preps. A world where expensive cloning reagents were unnecessary. A world where DNA and protein extraction and purification could be accomplished in a single microcentrifuge tube. We did, and so we created Clonebots, an automated approach to synthetic biology.
Clonebots seeks to simplify and automate common laboratory procedures to protocols involving only liquid handling steps that can be readily performed by robots. We will accomplish this by genetically encoding many of the required steps in E. coli, thereby allowing the cells to perform the required protocols in vivo. We worked on a wide variety of devices for these projects, and the details about our devices are described throughout this wiki. Most of our devices are associated with biologically encoding the reactions needed for standard assembly.
Layered Assembly
Our efforts to genetically encode standard assembly protocols centered around the layered assembly scheme used by the Anderson Lab at UC Berkeley. Layered assembly is a straight-forward and robust method for combining two or more basic parts into a destination vector. It involves three basic layers of vectors: Entry, Assembly and Destination. Parts are passed between the layers using Gateway reactions (normal arrows) and are assembled within the Assembly layer using 2ab reactions (Chevron arrows), a variation on Biobrick standard assembly. Entry and Assembly vectors are standardized for ease of use, while the Destination vectors can be tailored to a specific experiment or assay.
Entry Layer
Biobrick parts are created in an entry vector. The Entry vector contains a Spectinomycin antibiotic resistance marker and Biobrick restriction sites (EcoRI, XbaI, and SpeI for the BBa standard; EcoRI, BglII, and BamHI for the BBb1 standard). The restriction sites are flanked by attR1 and attR2 recombination sites. Since the entry plasmid contains attR recombination sites, the basic part can easily be transferred into one of six different assembly vectors using the Gateway cloning scheme:
Assembly Layer
The Assembly layer is used to combine two or more basic parts in a specified order to create a composite part.
There are six assembly plasmids which contain two of three different antibiotic resistance markers (kanamycin, ampicillin and Chloramphenicol) separated by a XhoI restriction site. The basic parts are flanked by BglII and BamHI restriction sites.
The choice of antibiotic resistance markers in the assembly plasmids is predetermined such that once the two plasmids recombine, the new plasmid will have a combination of markers from the two assembly vectors. Although there are four possible products of this reaction, only one will have both of the correct antibiotic resistance markers.
The Clotho program developed by the UC Berkeley Comp team generates an assembly tree that minimizes the number of reactions required to make a composite part.
The Assembly plasmids are transformed into cells that are engineered to methylate either BamHI or BglII restriction sites. Part A is transformed into a "lefty" cell that is methylated on BglII restriction sites while Part B is transformed into a "righty" cell that is methylated on BamHI restriction sites.
In a single microcentrifuge tube, lefty and righty plasmid DNA is combined, along with BamHI, BglII, XhoI restriction enzymes and ligase. Since restriction enzymes will not cut methylated DNA, the BglII restriction site in the lefty cell and the BamHI restriction site in the righty cell are blocked from digestion. The BamHI site in lefty and the BglII site in righty, and the XhoI sites in both plasmids will be cut. Ligase will join the XhoI sites and the BglII/BamHI complimentary sticky ends to create a composite part with a different combination of antibiotic resistance genes than either parent plasmid.
The assembly reaction can be repeated to combine a number of basic parts in a desired order.
Once the composite part is complete, it is transferred to a destination plasmid.
Destination Layer
Completed composite parts are transferred from the assembly layer to the destination layer using the Gateway cloning scheme. The destination plasmid is tailored to a specific experiment or assay. This allows a single composite part to be transferred to several different destination plasmids for further experimentation or characterization.
Issues with Current Methods
- Current protocols for standard assembly work pretty well, but assembly of basic parts remains the time and cost-limiting aspect of synthetic biology research. Clonebots will help us simplify standard assembly procedures making them more amenable to large-scale automation.
- Gateway reactions, used for transferring basic parts into the assembly plasmids and transferring composite parts into destination plasmids, are expensive ($8.65/reaction).
- Mini-preps are time-consuming and expensive.
- People are more prone to make mistakes, i.e. switch tubes, use the wrong enzymes, etc. than robots.
Clonebots Solutions
- Engineer cells to express their own integrase (Int), excisionase (Xis), and integration host factor (IHF) enzymes to perform in vivo Gateway reactions.
- Eliminate mini-prep steps from cloning protocols by using our lysis device and in vivo assembly.
- Engineer cells and plasmids to express their own BamHI, BglII, Cre, and ligase enzymes so that assembly reactions in cell lysate and in vivo are possible.
- Develop protocols that involve only liquid handling steps, so that cloning can be automated, requiring less money and labor.
 "
"