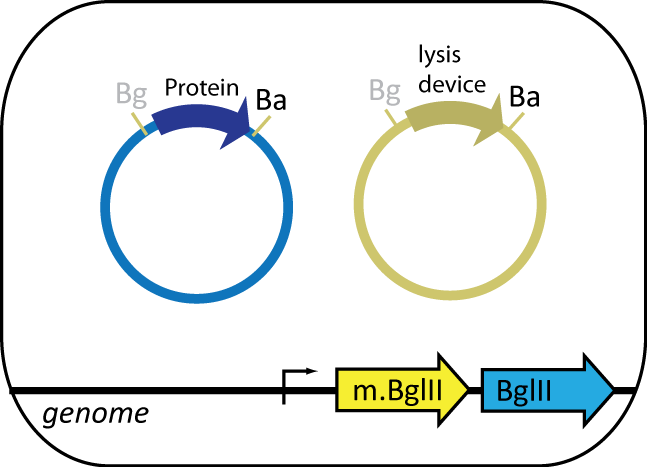Team:UC Berkeley/ProteinPurification
From 2008.igem.org
| (20 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
{{UCBmain}} | {{UCBmain}} | ||
| - | + | <div style="text-align: center;"><font size="6">'''Protein Purification'''</font></div><br> | |
=='''Introduction'''== | =='''Introduction'''== | ||
| - | Protein purification involves a series of step to isolate the protein of interest. Purifying a protein from E.coli first includes an extraction step, which brings the protein into solution. There are many physical or chemical methods from which to select for extraction. However, many times the | + | Protein purification involves a series of step to isolate the protein of interest. Purifying a protein from E.coli first includes an extraction step, which brings the protein into solution. There are many physical or chemical methods from which to select for extraction. However, many times the protein of interest may be too fragile such that putting the protein through harsh extractions will permanently damage the protein, resulting in low yield. The proposed solution for this is to transform an inducible λ phage lysis device. This allows complete control over lysis rate. In addition, using a natural form of lysis is much gentler on the cells and proteins, which will lead to greater success of protein extraction and purifcation. |
Additional steps can be taken to lower the background that comes from genomic DNA and RNA that flow into solution from extraction. Engineering the ability to make the restriction enzymes BamHI and BgIII and ribonuclease barnase will greatly reduce background. | Additional steps can be taken to lower the background that comes from genomic DNA and RNA that flow into solution from extraction. Engineering the ability to make the restriction enzymes BamHI and BgIII and ribonuclease barnase will greatly reduce background. | ||
| Line 16: | Line 17: | ||
[[Image:ucbigemppexpression.png|300 px]] | [[Image:ucbigemppexpression.png|300 px]] | ||
| + | |||
'''Helper Cell''' | '''Helper Cell''' | ||
| - | The helper cell contains parts the serve to reduce background during protein purification. The cell has BamHI methyltransferse engineered into its genome at the φ80 site. The plasmid in this cell contains BamHI, barnase and barstar. BamHI is used to degrade the genomic DNA from the expression cell. Barnase is used to degrade RNA of the expression cell and other helper cells when lysed. Barstar prevents barnase from | + | The helper cell contains parts the serve to reduce background during protein purification. The cell has BamHI methyltransferse engineered into its genome at the φ80 site. The plasmid in this cell contains BamHI, barnase and barstar. BamHI is used to degrade the genomic DNA from the expression cell. Barnase is used to degrade RNA of the expression cell and other helper cells when lysed. Barstar prevents barnase from destroying the cell's RNA. Like the expression cell, the helper cells contains the same inducible lysis device. |
| + | |||
[[Image:ucbigempphelper.png|300 px]] | [[Image:ucbigempphelper.png|300 px]] | ||
| + | |||
'''Protein of Interest''' | '''Protein of Interest''' | ||
The protein of interest is on a plasmid that is transformed into the expression cell. The protein will have a protein tag. | The protein of interest is on a plasmid that is transformed into the expression cell. The protein will have a protein tag. | ||
| + | |||
[[Image:ucbproteinofinterestplasmid.png]] | [[Image:ucbproteinofinterestplasmid.png]] | ||
| + | |||
'''Tags''' | '''Tags''' | ||
| - | Four tags were made: AP, HA, myc and FLAG. AP binds to streptavidin, Ha binds to hemagglutinin. | + | |
| + | Four tags were made: AP, HA, myc and FLAG. AP binds to streptavidin, Ha binds to hemagglutinin, myc binds to c-myc and FLAG binds to anti-DDK antibodies. To test the tags, composite parts of the following construction Pbad.rbs_pelB.phoA.tag.b1006 were made. rbs_pelB is one of the four [https://2008.igem.org/Team:UC_Berkeley/Notebook/Aron_Lau/prepro prepro sequences] that sends a protein to the periplasm. Additional information on how the the tags is in the [https://2008.igem.org/Team:UC_Berkeley/ProteinPurification#Tags_and_Testing experimental section]. | ||
| + | |||
[[Image:ucbigemtag.png]] | [[Image:ucbigemtag.png]] | ||
| Line 38: | Line 46: | ||
The protein purification strategy involves growing up cultures of expression cells and helper cells. Combining the two cultures and lysing them will result in a solution of proteins, among which contains the protein of interest and cellular junk minus the RNA (degraded by barnase) and DNA (degraded by one of the two restriction enzymes). At this point, using the tag on the protein, the protein will be taken out of solution by taking advantage of the selective interaction between the tag and the antibody that binds to it. | The protein purification strategy involves growing up cultures of expression cells and helper cells. Combining the two cultures and lysing them will result in a solution of proteins, among which contains the protein of interest and cellular junk minus the RNA (degraded by barnase) and DNA (degraded by one of the two restriction enzymes). At this point, using the tag on the protein, the protein will be taken out of solution by taking advantage of the selective interaction between the tag and the antibody that binds to it. | ||
| + | =='''Tags and Testing'''== | ||
| + | To test the tags, an ELISA was performed. First, the antibodies were diluted to a final concentration of 20 μg/ml in PBS. 50 ul of this antibody solution is added to a NUNC maxisorp plate and incubated for an hour at 37 degree C. After an hour, the plate was washed with [https://2008.igem.org/Team:UC_Berkeley/Notebook/Aron_Lau/material#Wash_Solution.281L.29 wash solution] using a plate washer. 200ul of [https://2008.igem.org/Team:UC_Berkeley/Notebook/Aron_Lau/material#Blocking_Solution.28500_mL.29 blocking solution] was added to the plate and incubated for an hour at 37 degree C. Meanwhile, because the phoA.tag complex is sent to the periplasm with the pelB sequence, a [https://2008.igem.org/Team:UC_Berkeley/Protocols#Periplasmic_Prep periplasmic prep] was performed to get a solution of the phoA.tag complex and was subsequently diluted down to the working concentration with PBS. After an hour of blocking, the plate was again washed with wash buffer using the plate washer. The 200ul of phoA.tag dilution was added to the plate and was incubated at 37 degree C for an hour. The plate was washed afterward and 100ul of PNP was then added to the wells, observing for a yellow color. | ||
| - | == | + | <html> |
| - | + | <a href="https://2008.igem.org/Template:Team:UC_Berkeley/Notebook/MT_anthropological_narrative" class="titleIcon"> | |
| - | + | <img align=right src="https://static.igem.org/mediawiki/2008/9/9a/GreyNext.png"> | |
| - | + | </a> | |
| + | </html> | ||
Latest revision as of 03:52, 30 October 2008
Introduction
Protein purification involves a series of step to isolate the protein of interest. Purifying a protein from E.coli first includes an extraction step, which brings the protein into solution. There are many physical or chemical methods from which to select for extraction. However, many times the protein of interest may be too fragile such that putting the protein through harsh extractions will permanently damage the protein, resulting in low yield. The proposed solution for this is to transform an inducible λ phage lysis device. This allows complete control over lysis rate. In addition, using a natural form of lysis is much gentler on the cells and proteins, which will lead to greater success of protein extraction and purifcation.
Additional steps can be taken to lower the background that comes from genomic DNA and RNA that flow into solution from extraction. Engineering the ability to make the restriction enzymes BamHI and BgIII and ribonuclease barnase will greatly reduce background.
To identify the protein in the large soup of protein, the four protein tags AP, HA, myc and flag were made. These tags can be attached at the terminus of the protein strands and their selective interactions between antibodies and themselves will make the tagged proteins easier to isolate.
Devices
Expression Cell
The expression cell, containing the plasmid coding for the protein of interest, features BgIII restriction enzyme and BgIII methyltransferase parts engineered into the genome. BgIII, integrated at the HK022 site, is used to cut up the genome of the helper cell. BgIII methyltransferase, integrated at φ80 site, prevents BgIII from cutting up the cell's own genome. The expression cell also contains an inducible lysis device that lyses the cell upon induction.
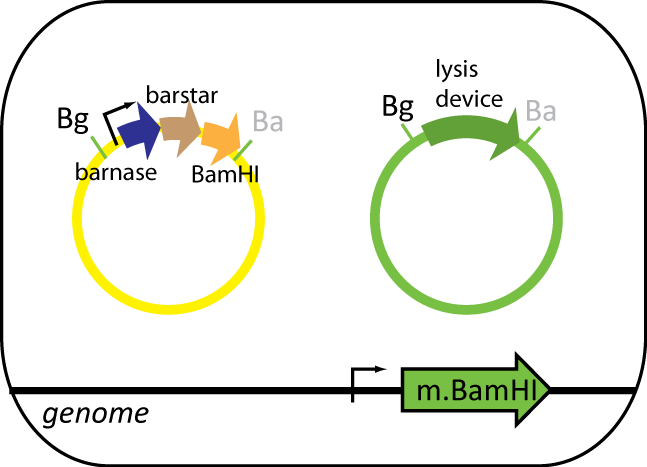
Helper Cell
The helper cell contains parts the serve to reduce background during protein purification. The cell has BamHI methyltransferse engineered into its genome at the φ80 site. The plasmid in this cell contains BamHI, barnase and barstar. BamHI is used to degrade the genomic DNA from the expression cell. Barnase is used to degrade RNA of the expression cell and other helper cells when lysed. Barstar prevents barnase from destroying the cell's RNA. Like the expression cell, the helper cells contains the same inducible lysis device.
Protein of Interest
The protein of interest is on a plasmid that is transformed into the expression cell. The protein will have a protein tag.
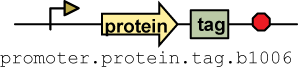
Tags
Four tags were made: AP, HA, myc and FLAG. AP binds to streptavidin, Ha binds to hemagglutinin, myc binds to c-myc and FLAG binds to anti-DDK antibodies. To test the tags, composite parts of the following construction Pbad.rbs_pelB.phoA.tag.b1006 were made. rbs_pelB is one of the four prepro sequences that sends a protein to the periplasm. Additional information on how the the tags is in the experimental section.
Strategy
The protein purification strategy involves growing up cultures of expression cells and helper cells. Combining the two cultures and lysing them will result in a solution of proteins, among which contains the protein of interest and cellular junk minus the RNA (degraded by barnase) and DNA (degraded by one of the two restriction enzymes). At this point, using the tag on the protein, the protein will be taken out of solution by taking advantage of the selective interaction between the tag and the antibody that binds to it.
Tags and Testing
To test the tags, an ELISA was performed. First, the antibodies were diluted to a final concentration of 20 μg/ml in PBS. 50 ul of this antibody solution is added to a NUNC maxisorp plate and incubated for an hour at 37 degree C. After an hour, the plate was washed with wash solution using a plate washer. 200ul of blocking solution was added to the plate and incubated for an hour at 37 degree C. Meanwhile, because the phoA.tag complex is sent to the periplasm with the pelB sequence, a periplasmic prep was performed to get a solution of the phoA.tag complex and was subsequently diluted down to the working concentration with PBS. After an hour of blocking, the plate was again washed with wash buffer using the plate washer. The 200ul of phoA.tag dilution was added to the plate and was incubated at 37 degree C for an hour. The plate was washed afterward and 100ul of PNP was then added to the wells, observing for a yellow color.
 "
"