Team:UNIPV-Pavia/Notebook/Week8
From 2008.igem.org
(Difference between revisions)
| Line 163: | Line 163: | ||
'''07/9/08''' | '''07/9/08''' | ||
<br> | <br> | ||
| + | *We transformed the 9 ligations (2 µl) and plated transformed bacteria. We incubated plates at 37°C ovenight. | ||
| + | |||
*Glycerol stocks for BBa_B1006 and '''BBa_B0030'''-BBa_C0078. | *Glycerol stocks for BBa_B1006 and '''BBa_B0030'''-BBa_C0078. | ||
*Miniprep for BBa_B1006 and '''BBa_B0030'''-BBa_C0078. | *Miniprep for BBa_B1006 and '''BBa_B0030'''-BBa_C0078. | ||
| + | |||
| + | *Plasmid digestion: | ||
| + | {|cellpadding="20px" | ||
| + | |BBa_B1006 (E-X) | ||
| + | |'''BBa_B0030'''-BBa_C0078 (E-S) | ||
| + | |} | ||
| + | |||
| + | *Gel run/cut/gel extraction for '''BBa_B0030'''-BBa_C0078 (E-S). | ||
| + | |||
| + | *DNA precipitation with sodium acetate for BBa_B1006 (E-X). | ||
| + | |||
| + | *Ligation: BBa_B0030-BBa_C0078-'''BBa_B1006''' | ||
| + | |||
| + | *We incubated ligation at 16°C overnight. | ||
| + | |||
| + | <br><br> | ||
| + | '''07/10/08''' | ||
| + | <br> | ||
| + | *We transformed BBa_B0030-BBa_C0078-'''BBa_B1006''' ligation (2 µl) and plated transformed bacteria. We incubated the plate at 37°C ovenight. | ||
| + | |||
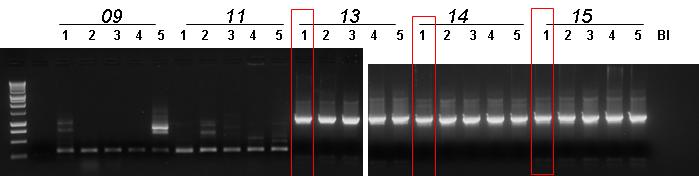
| + | *All the ligation plates showed colonies! There were carpets, but we could pick up some single colonies for colony PCR. | ||
| + | |||
| + | *Colony PCR for | ||
Revision as of 11:28, 20 July 2008
Notebook
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
|---|---|---|---|---|---|---|
| Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 |
Week 8: 07/7/08 - 07/12/08
07/7/08
- Colony PCR (5 colonies for each plate) for:
- BBa_J23100-BBa_B0030-BBa_C0012 (for the second time, we hoped to find true positive colonies)
- BBa_J23100-BBa_B0030-BBa_I15010 (for the second time, we hoped to find true positive colonies)
- BBa_B0030-BBa_E0040-BBa_B1006
- BBa_B0030-BBa_C0051-BBa_B0030
- BBa_B0030-BBa_E1010-BBa_B1006
- Gel results:
- No true positives for BBa_J23100-BBa_B0030-BBa_C0012
- No true positives for BBa_J23100-BBa_B0030-BBa_I15010
- Non pure true positives for BBa_B0030-BBa_E0040-BBa_B1006
- Pure true positives for BBa_B0030-BBa_C0051-BBa_B0030
- Non pure true positives for BBa_B0030-BBa_E1010-BBa_B1006
- We chose to keep the first colony for all the 3 working ligation plates.
- NOTE: BBa_B0030-BBa_E0040-BBa_B1006 and BBa_B0030-BBa_E1010-BBa_B1006 are final parts; we decided to sequence these 2 parts even if gel showed a weak contamination: sequencing results will tell us if there are false positive plasmids in those colonies or if the contamination was only a PCR contamination.
- We infected 9 ml LB + suitable antibiotic with 30 µl of these glycerol stocks:
| BBa_B0030 | BBa_I15010 | BBa_B0030-BBa_E0040-BBa_B1006 (1) | BBa_B0030-BBa_E1010-BBa_B1006 (1) |
| BBa_C0012 | BBa_R0062 | BBa_B0030-BBa_C0051-BBa_B0030 | BBa_J23100-BBa_B0030 |
- We incubated the 8 cultures at 37°C, 220 rpm overnight.
- Tomorrow we will be ready to perform NINE LIGATIONS...(@_@!)
07/8/08
- We received sequencing results for BBa_B0030-BBa_C0078: sequence showed an extra DNA fragment between BBa_C0078 and Suffix...We decided to ignore it because there was a stop codon before that fragment.
- Glycerol stocks for:
| BBa_B0030 | BBa_I15010 | BBa_B0030-BBa_E0040-BBa_B1006 (1) | BBa_B0030-BBa_E1010-BBa_B1006 (1) |
| BBa_C0012 | BBa_R0062 | BBa_B0030-BBa_C0051-BBa_B0030 | BBa_J23100-BBa_B0030 |
- Miniprep for these parts.
- Plasmid digestion for:
| BBa_B0030 (S-P) | BBa_I15010 (X-P) | BBa_B0030-BBa_E0040-BBa_B1006 (1) (E-X) | BBa_B0030-BBa_E1010-BBa_B1006 (1) (E-X) |
| BBa_C0012 (X-P) | BBa_R0062 (E-X) | BBa_B0030-BBa_C0051-BBa_B0030 (S-P) | BBa_J23100-BBa_B0030 (E-S) |
| BBa_J23100-BBa_B0030-BBa_C0040 (E-S) | BBa_R0051-BBa_B0030-BBa_C0062 (E-S) | BBa_B0030-BBa_C0061-BBa_B1006 (E-S) |
- Gel run/cut/gel extraction for:
| BBa_I15010 (X-P) | BBa_J23100-BBa_B0030 (E-S) | BBa_J23100-BBa_B0030-BBa_C0040 (E-S) |
| BBa_C0012 (X-P) | BBa_R0051-BBa_B0030-BBa_C0062 (E-S) | BBa_B0030-BBa_C0061-BBa_B1006 (E-S) |
- DNA precipitation with sodium acetate for:
| BBa_B0030 (S-P) | BBa_B0030-BBa_E0040-BBa_B1006 (1) (E-X) | BBa_B0030-BBa_E1010-BBa_B1006 (1) (E-X) |
| BBa_R0062 (E-X) | BBa_B0030-BBa_C0051-BBa_B0030 (S-P) |
- (We already had BBa_I15009(X-P) and BBa_B1006(E-X))
- Ligations:
- BBa_B0030-BBa_I15009
- BBa_J23100-BBa_B0030-BBa_C0040-BBa_B1006
- BBa_R0051-BBa_B0030-BBa_C0062-BBa_B1006
- BBa_B0030-BBa_C0051-BBa_B0030-BBa_C0079
- BBa_B0030-BBa_C0061-BBa_B1006-BBa_R0062
- BBa_J23100-BBa_B0030-BBa_C0012 (again)
- BBa_J23100-BBa_B0030-BBa_I15010 (again)
- BBa_J23100-BBa_B0030-BBa_E0040-BBa_B1006 (to test the part)
- BBa_J23100-BBa_B0030-BBa_E1010-BBa_B1006 (to test the part)
- We incubated ligations at 16°C overnight.
- We infected 9 ml LB + Amp with 30 µl of BBa_B1006 and BBa_B0030-BBa_C0078 glycerol stocks. We incubated the 2 cultures at 37°C, 220 rpm ovenight.
07/9/08
- We transformed the 9 ligations (2 µl) and plated transformed bacteria. We incubated plates at 37°C ovenight.
- Glycerol stocks for BBa_B1006 and BBa_B0030-BBa_C0078.
- Miniprep for BBa_B1006 and BBa_B0030-BBa_C0078.
- Plasmid digestion:
| BBa_B1006 (E-X) | BBa_B0030-BBa_C0078 (E-S) |
- Gel run/cut/gel extraction for BBa_B0030-BBa_C0078 (E-S).
- DNA precipitation with sodium acetate for BBa_B1006 (E-X).
- Ligation: BBa_B0030-BBa_C0078-BBa_B1006
- We incubated ligation at 16°C overnight.
07/10/08
- We transformed BBa_B0030-BBa_C0078-BBa_B1006 ligation (2 µl) and plated transformed bacteria. We incubated the plate at 37°C ovenight.
- All the ligation plates showed colonies! There were carpets, but we could pick up some single colonies for colony PCR.
- Colony PCR for
 "
"