Team:Freiburg/Project
From 2008.igem.org
m |
|||
| Line 23: | Line 23: | ||
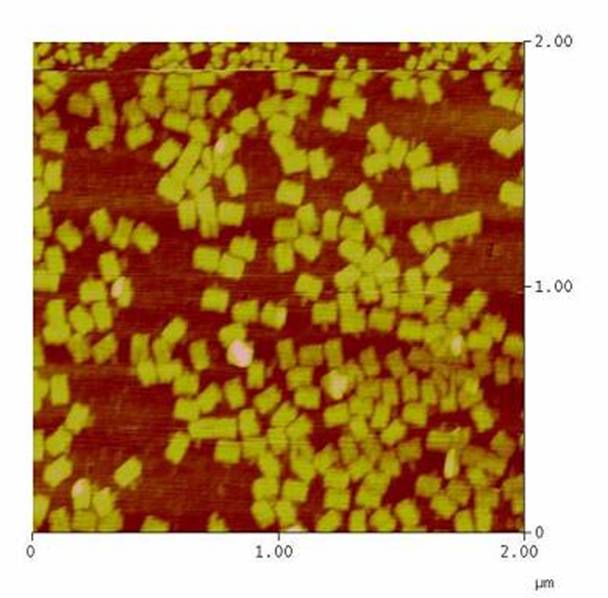
- Can we improve DNA origami assembly yield by varying the staple oligonucleotide to scaffold DNA ratio?<br> | - Can we improve DNA origami assembly yield by varying the staple oligonucleotide to scaffold DNA ratio?<br> | ||
- Can we find buffer conditions, which mediate cell viability and origami stability?<br> | - Can we find buffer conditions, which mediate cell viability and origami stability?<br> | ||
| - | - Can we downsize a | + | - Can we downsize a Ca<sup>2+</sup> release assay to fit the sample sizes of our precious DNA origami?<br> |
- Do our constructs express and then localize in the membrane of eukaryotic cells?<br> | - Do our constructs express and then localize in the membrane of eukaryotic cells?<br> | ||
- Do DNA origamis bind to cells?<br> | - Do DNA origamis bind to cells?<br> | ||
| Line 41: | Line 41: | ||
<!--[[Team:Freiburg_Cell Culture|Cell Culture]]</h3><br>--> | <!--[[Team:Freiburg_Cell Culture|Cell Culture]]</h3><br>--> | ||
[[Team:Freiburg_Transfection and Synthetic Receptor|Transfection and Synthetic Receptor Activation]]<br> | [[Team:Freiburg_Transfection and Synthetic Receptor|Transfection and Synthetic Receptor Activation]]<br> | ||
| - | [[Team:Freiburg_Calcium Imaging|Cell Stability, | + | [[Team:Freiburg_Calcium Imaging|Cell Stability, Ca<sup>2+</sup> Signaling and DNA-Origami-Binding]]<br><br> |
<h2>'''Highlights'''</h2> | <h2>'''Highlights'''</h2> | ||
| Line 114: | Line 114: | ||
</td> | </td> | ||
<td> | <td> | ||
| - | <h4>[[Team:Freiburg_Calcium Imaging|Cell Stability, | + | <h4>[[Team:Freiburg_Calcium Imaging|Cell Stability, Ca<sup>2+</sup> Signaling and DNA-Origami-Binding]]</h4> |
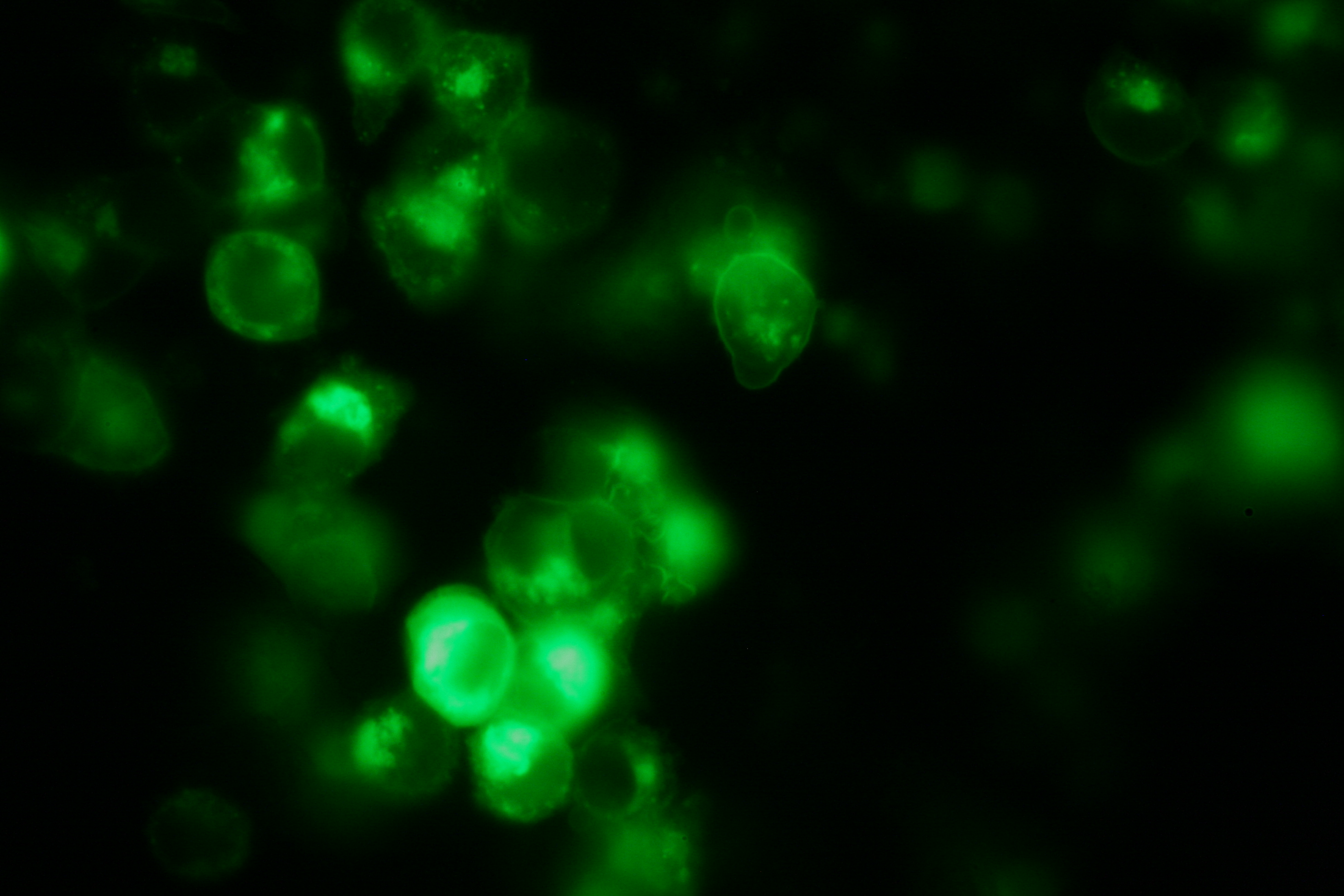
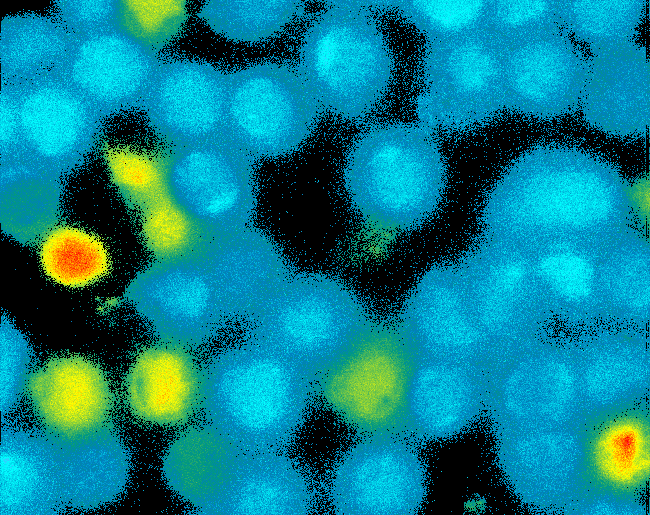
Due to the small total amounts of DNA-Origami we could not use FACS to measure calcium influx and, thus, T-cell activation. The alternative we found were "Nano-Slides" for inverse fluorescence microscopy; T-cells were immobilized on the poly-L-Lysine coated slides and stimulated directly under the microscope. This procedure allowed real-time observation of calcium-influx. <br> | Due to the small total amounts of DNA-Origami we could not use FACS to measure calcium influx and, thus, T-cell activation. The alternative we found were "Nano-Slides" for inverse fluorescence microscopy; T-cells were immobilized on the poly-L-Lysine coated slides and stimulated directly under the microscope. This procedure allowed real-time observation of calcium-influx. <br> | ||
</td> | </td> | ||
Revision as of 01:04, 30 October 2008
|
Project Report |
_project report
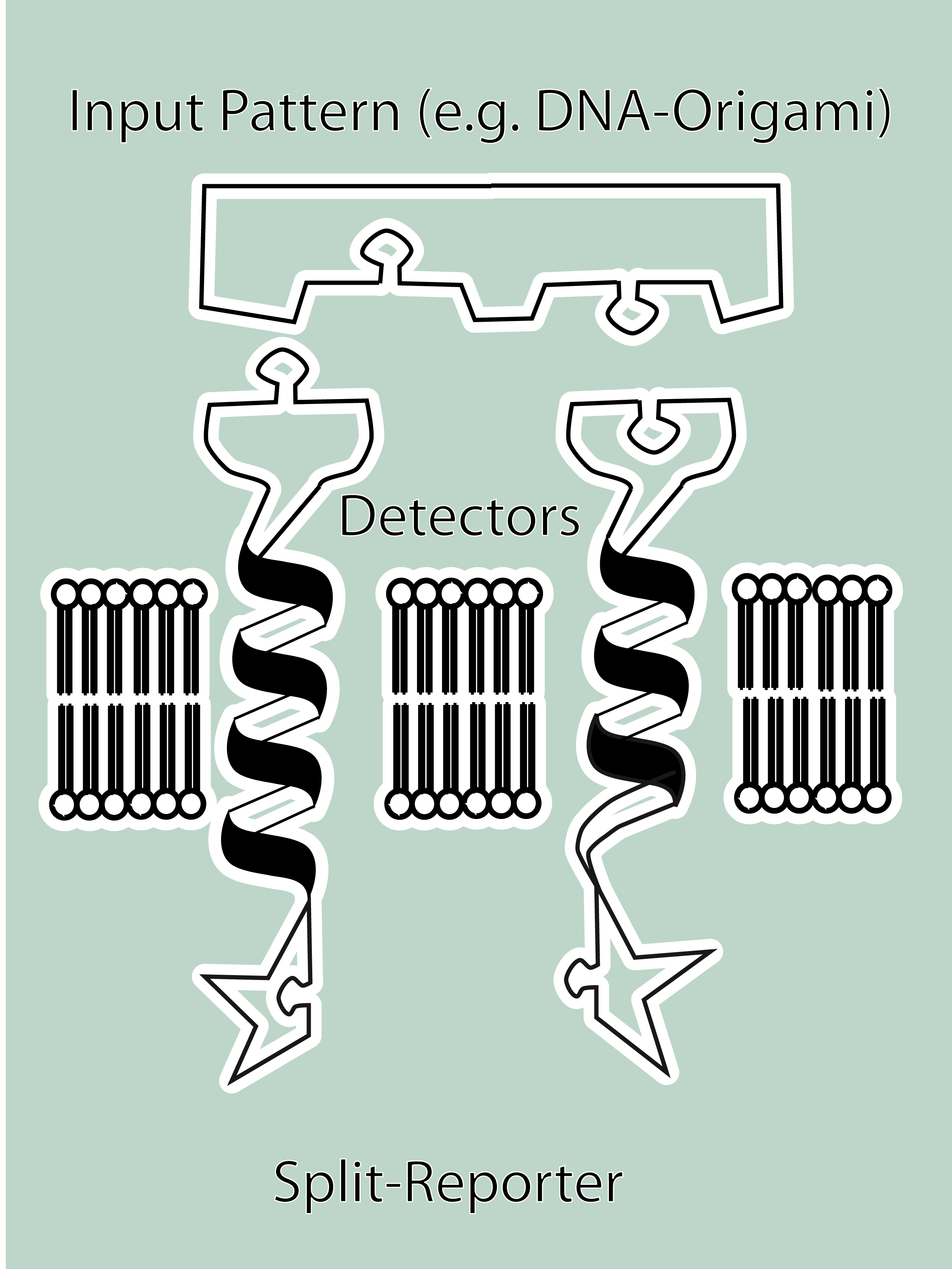
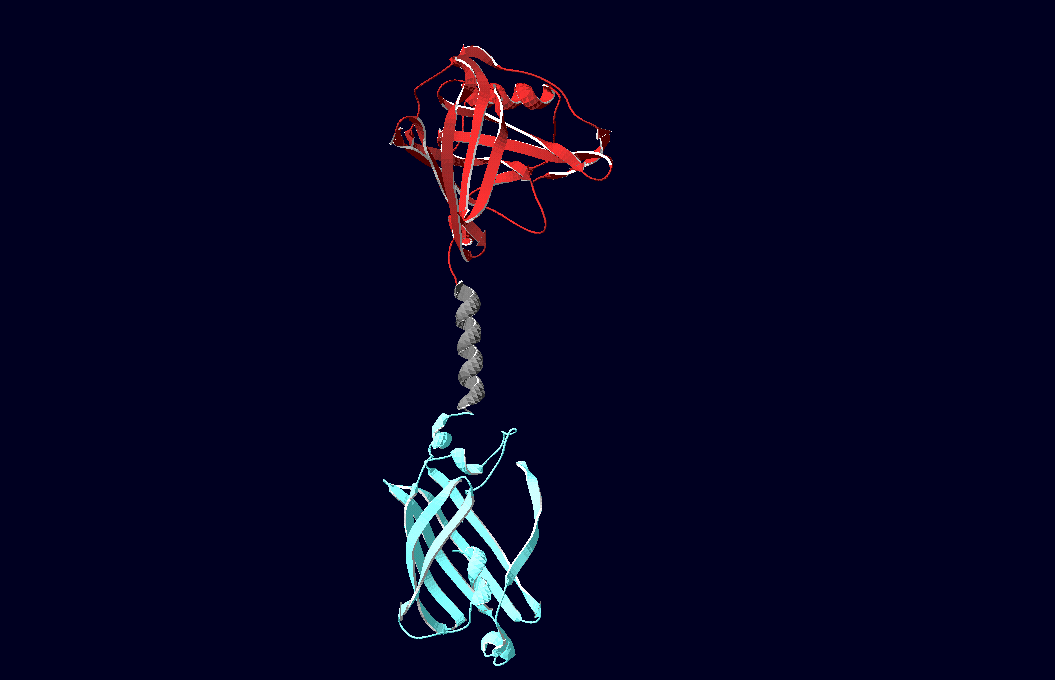
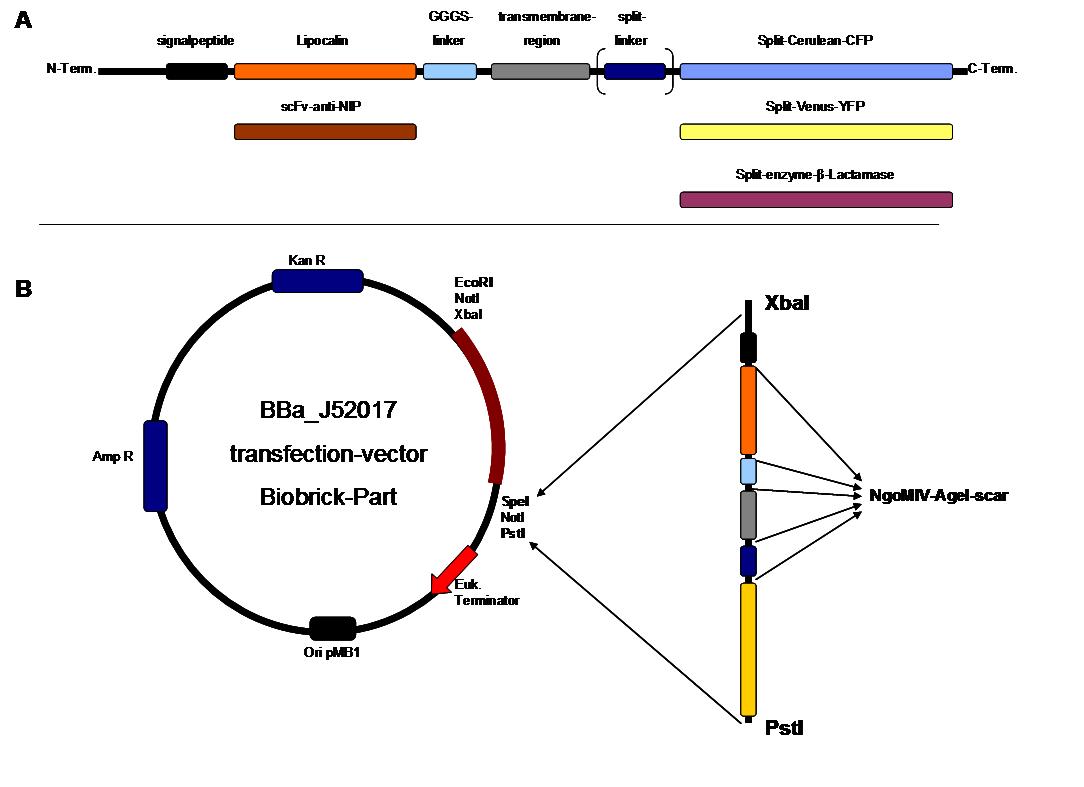
SummaryModular Synthetic Receptor System In the following we provide a summary of our project and display the highlights of our achievements. For a more detailed view please see the reports of each subproject. The general goal of the Freiburg 2008 team is to establish a synthetic transmembrane receptor system comprising the extracellular input devices, the extracellular receiver domain, the transducer to the cytosol and the reporter/executor in the cytosol. To facilitate binding, we used a single-chain Fv antibody fragment and a designed anticalin. Both bind haptens (nitro-jodo-phenol and fluorescein) which can be conjugated to specific oligonucleotides of the DNA-Origami or scaffold proteins. Transduction to the cytosol is mediated by a single transmembrane helix. As intracellular reporters we employ split proteins. Here, we used the split lactamase from the iGEM2007 Freiburg project as well as the split fluorescent proteins CFP/cerulean and YFP/venus. To provide steric flexibility and reach of our binding and reporting modules we interspersed linkers between each functional domain. All parts submitted feature full BioBrick-compatibility and in addition allow for the construction of fusion proteins due to extended Biobrick 3.0 standard. Cloning was done in E. coli. However, we test our receptors in human or mouse cells/chassis. Thus, to direct our constructs to the cell surface we attached a eukaryotic signal peptide taken from hEGF-R to the N-terminus of each synthetic receptor. In addition to our designed synthetic receptors, we initially also employed an existing T-cell line displaying a TCR fused to a single chain. To reach our goal within the short given time frame we started several subprojects in parallel. Our subprojects listed here are defined along these projects. Besides designing and cloning parts we spent quite some time on establishing and analyzing the biological experiments. Initially we tested interfacing of DNA origami with cells and the spatial control via DNA origami using an existing cell line expressing the scFv fused to a T-cell receptor. In our experiments we addressed the following questions: - Can we improve DNA origami assembly yield by varying the staple oligonucleotide to scaffold DNA ratio? In our modeling analyses we constructed various sets of differential equations for our synthetic receptors and predicted split protein activation behaviour. The labs of Kristian Müller and Katja Arndt provided all basic technology and support to get started. For advanced analyses several labs of the University of Freiburg and the Max-Planck-Institute of Immunobiology granted access to their instrumentation (e.g. atomic force micoscopy, confocal microscopy, FACS).
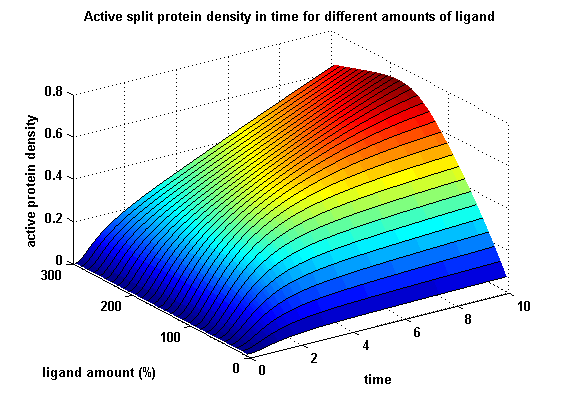
SubprojectsModeling Highlights
|
 "
"