|
Home
The Team
Project Report
Parts
Modeling
Notebook
Safety
CoLABoration
|
_project report
Introduction:
This year´s main project is the attempt to create an "artificial receptor-system", featuring extra- and intracellular modules as well as suitable transmembrane regions.
The intracellular domaine of our receptor-device is build by halves of split reporter-proteins that can reassemble and will then produce readable output, e. g. fluorescence.
Each one of these protein-halves is connected to its extracellular domaine by a single-span transmembrane-helix.
The extracellular or detecting domaine consists of a protein or peptide with the ability to bind a certain molecule.
Now, if a system with two matching receptors is presented these molecules in a strict, pairwise spatial arrangement, the receptor-devices are brought together,
the split reporter-protein reassembles inside the cell and the output can be detected.
We employ so-called "Origami-DNA" to create the exactly defined molecule-patterns that are needed to activate our receptors.
One of the main inspirations that lead to the idea of creating a synthetic receptor-like fusion protein is based on an immunologic study on the signaling pathway of the T-Cell-Receptor (TCR) that has been performed by Wolfgang Schamel at the Max-Planck-Institute for immunology, Freiburg.
In this study he used modified TCRs with Fab-Fragment-singlechains of Anti-NIP –Antibodies fused to their ß-domaines by a flexible linker that would present them on the cell´s surface.
This modification would allow to investigate the influence of receptor-clustering on the intensity of the cell-signaling. It could been shown that there is a relation between the clustering of the antigen and, thus, of the receptors by presenting various peptides with certain amounts and arrangements of NIP-molecules as stimulus.
Anyway, this experiment was restricted by the one-dimensionality of the antigen-fused peptides; at this point, the Origami-DNA comes into play:
Paul Rothemund had discovered that it is possible to shape M13-Phage single-strand-DNA simply adding oligonucleotides that would work as „brackets“ when complementing the long single-strand. In this way, one can generate DNA-squares of a certain size with „nods“ at certain distances.
One member of our team, Daniel Hautzinger, has recently finished his diploma-thesis on Origami-DNA and the possibilities of generating patterns on these square surfaces by modifying the Oligo-nucleotides that build up the nod-points.
As the antigen NIP can as well be fused to these oligos, it was now possible to present strictly defined two-dimensional antigen-patterns to T-Cells carrying the modified receptors mentioned above.
This, again, made us come up with the idea of a transmembrane-fusion-protein that could be spatially arranged from outside the cell by the pattern on the Origami-DNA-surface.
Of course, the first extracellular domaine we had in mind was the anti-NIP-singlechain Schamel had used with his receptors. The first intracellular domaines should consist of the split-lactamase-halfes we designed as parts for last year´s iGEM, as this enzyme´s activity can be regained by complementation of the halves and detected by a fluorescent substrate.
Now, we were looking for a single-span-transmembrane-protein; as the domaines of the Epidermal-Growth-Factor Receptor are well known, we chose to employ it´s transmembrane-helix and the signal-peptide mediating the construct´s insertion into the membrane.
Further modules we had in mind were an Anti-Fluorescein-singlechain and a fluorescein-binding variety of Lipocalin by Arne Skerra as extracellular „detectors“ as well as the complementing halves of each one of the split-fluorophores „Cerulean“ (cyan) and „Venus“ (yellow) as intracellular „reporters“. These split-fluorophores feature cross-compatibility between the N- and C-terminal halves (green fluorescence), enabling our system to generate three different „outputs“ (yellow, blue, green) with only two molecules (NIP, FluA) building up the „input-pattern“ on the Origami-DNA-surface.
Subprojects:
Modeling
3D-Modeling
DNA-Origami
Cloning Strategy
Cell Culture
Transfection and Synthetic Receptor Activation
Cell Stability, Ca2+ Signaling and DNA-Origami-Binding
Highlights:
|
Modeling
The dimerization of the extracellular receptor domains is a important necessity for the functionality of our Modular Synthetic Receptor System. Presenting the system a stimulus in the form of spatial arranged ligands (nitro-iodo-phenol or fluorescein molecules), the extracellular domains dimerize, thus the corresponding intracellular parts such as the split lactamase halves or split fluorescent proteins complement to measureable output. To analyse the theoretical functionality due to dimerization, two receptor dimerization models (one T cell receptor model and one general receptor model) are introduced and discussed and a proper model for the Modular Synthetic Receptor System is constructed. It consists of 10 reaction kinetic equations, 9 ordinary differential equations including 25 variable parameters.
|
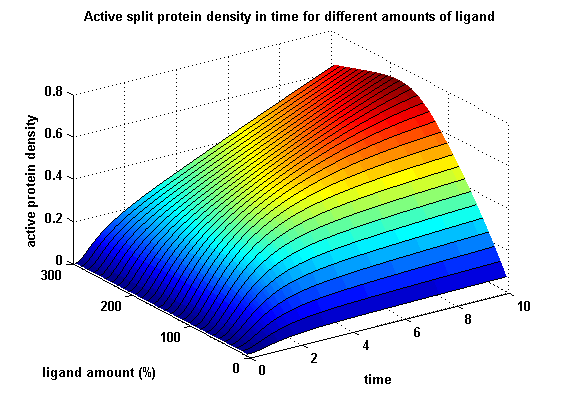 Figure 17: Split protein activity in time dependent on ligand amount
|
|
Transfection and Synthetic Receptor Activation
All of our constructs (see "Parts") were cloned successfully using our extended pre- and suffix. Transfection of 293T-cells has also been shown to work for most of our fusion proteins; so far, we could even show that at least our constructs with lipocalin FluA as extracellular domaine are integrated into the membrane (Fig.1, details see Transfection).
All transfections were carried out with part Bba_K157040, one of our composite parts consisting of the transfection vector Bba_J52017 and a CMV-promotor. Due to the limited time range we were not able to activate/dimerize any of the various possible "receptor"-pairs yet, but we are hoping to receive a positive result in that concern until the jamboree.
|
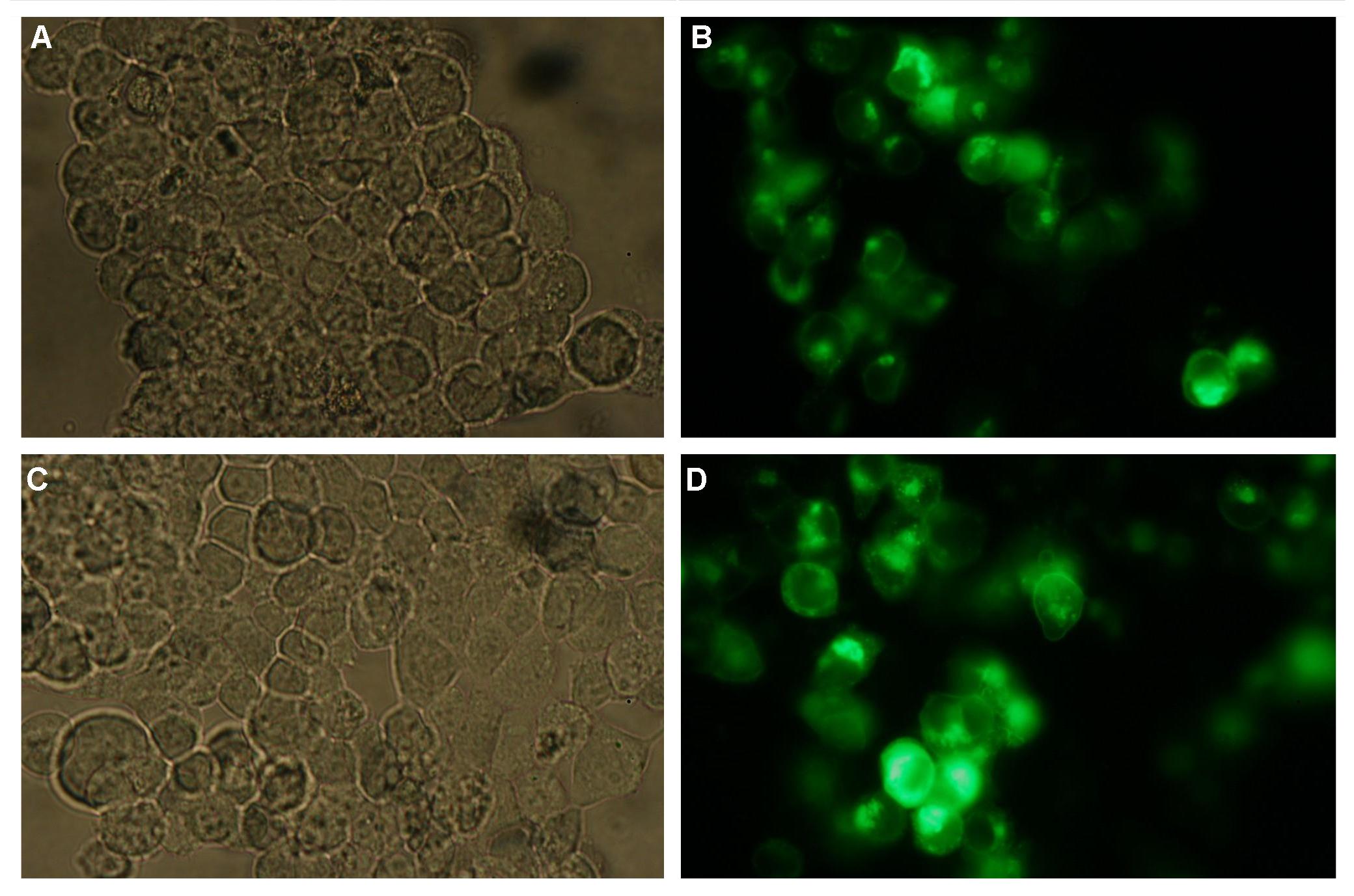 Fig.1:Transfection of 293T-Cells with one of our membrane-localized constructs (Part Bba_K157032-YFP) |
Literature:
Split-fluorophores:
-Chang-Deng Hu, Yurii Chinenov, Tom K. Kerppola: ”Visualization of Interactions among bZIP and Rel Family Proteins in Living Cells Using Bimolecular Fluorescence Complementation”, Molecular Cell, Vol. 9, 789–798, April, 2002
-Chang Deng Hu, Tom K. Kerppola: “Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis”, Nat Biotechnol. 2003 May; 21(5):539-545 (doi:10. 1038/nbt816)
-Tom K. Kerppola: “Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells”, Nat Protoc. 2006;1(3):1278-1286 (doi:10.1038/nprot.2006.201)
-Nagai, T. et al. “A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications” J. Biol. Chem. 276, 29188-29194, 2001
-Roger Y. Tsien et al. „Creating new fluorescent probes for cell biology“, Nature Biotechnology Reviews, Vol. 3, 906-918, 2002
LipocalinFluA:
-Gerald Beste, Frank S. Schmidt, Thomas Stibora and A. Skerra: “Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold“,Proc. Natl. Acad. Sci. USA Vol. 96, pp. 1898–1903, March 1999 Biochemistry
-Ingo P. Korndörfer, Gerald Beste and A. Skerra: “Crystallographic Analysis of an “Anticalin” With Tailored Specificity for Fluorescein Reveals High Structural Plasticity of the Lipocalin Loop Region”, PROTEINS: Structure, Function, and Bioinformatics 53:121–129 (2003)
DNA-Origami:
Paul W. K. Rothemund: Nature 440, 297-302 (16 March 2006)
Antibody B1-8:
-Ana Cumano and Klaus Rajewski: “Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP”, The EMBO Journal vol. 5 no.10 pp. 2459-2468, 1986
-D. Allen, T. Simon, F. Sablitzky, K. Rajewski and A. Cumano: “Antibody engineering for the analysis of affinity maturation of an anti-hapten response”, The EMBO Journal vol. 7 no.7 pp. 1995-2001, 1988
Modeling:
-Martin F. Bachmann, Michael Salzmann, Annette Oxenius and Pamela S. Ohashi: "Formation of TCR dimers/trimers as a crucial step for T cell activation", Eur. J. Immunol., 1998
-Martin F. Bachmann and Pamela S. Ohashi: "The role of T-cell receptor dimerization in T-cell activation", Review Immunology Today, Dezember 1999
-João Sousa and Jorge Carneiro: "A mathematical analysis of TCR serial triggering and down-regulation", Eur. J. Immunol., 2000
-Susana Minguet, Mahima Swamy, Balbino Alarcón, Immanuel F. Luescher and Wolfgang W.A. Schamel: "Full Activation of the T Cell Receptor requires Both Clustering and Conformational Changes at CD3", Immunity, 2006
|
 "
"