Team:Chiba/jk/β/week 2
From 2008.igem.org
>送受信班
Contents |
Week 2
>last week
24 August, 2008
- (22/8)--->Mini prep
- [http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007)
- [http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2006)
- [http://partsregistry.org/Part:BBa_J13002 BBa_J13002](2007)
- [http://partsregistry.org/Part:BBa_J13002 BBa_J13002](2006)
- --->Digestion test
- [http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007)
- [http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2006)
- [http://partsregistry.org/Part:BBa_J13002 BBa_J13002](2007)
- [http://partsregistry.org/Part:BBa_J13002 BBa_J13002](2006)
| Sample No. | 5 | 6 | 7 | 8 |
| [http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007) | 1 | - | - | - |
| [http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2006) | - | 1 | - | - |
| [http://partsregistry.org/Part:BBa_J13002 BBa_J13002](2007) | - | - | 1 | - |
| [http://partsregistry.org/Part:BBa_J13002 BBa_J13002](2007) | - | - | - | 1 |
| EcoRⅠ | 0.1 | 0.1 | 0.1 | 0.1 |
| Buffer 4 | 0.9 | 0.9 | 0.9 | 0.9 |
| dH2O | 8 | 8 | 8 | 8 |
| TOTAL | 10 | 10 | 10 | 10 |
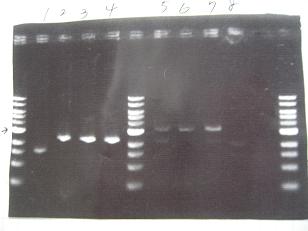
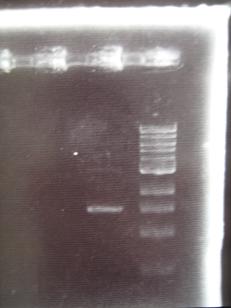
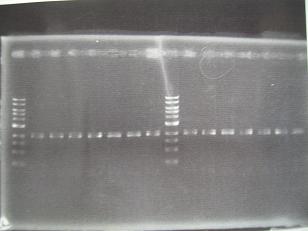
- --->Gel Check
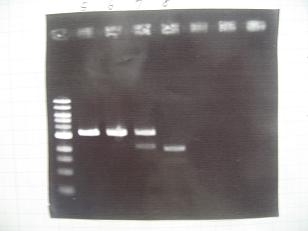
|
|
25 August, 2008
- (24/8)--->Digestion
- Double Digestion:[http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007)
- Double Digesiton:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007)
- Single Digestion:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007)
- [http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007)
| Sample No. | 1 | 2 | 3 | 4 |
| Sample DNA | 5 | 5 | 1 | 1 |
| EcoRⅠ | 0.5 | 0.5 | - | - |
| SpeⅠ | 0.5 | 0.5 | - | - |
| Buffer 1 | 1 | 1 | - | - |
| Buffer 3 | - | - | 1 | - |
| BSA | 1 | 1 | - | - |
| dH2O | 2 | 2 | 7.5 | - |
| TOTAL | 10 | 10 | 10 | 1 |
- --->Gel Check
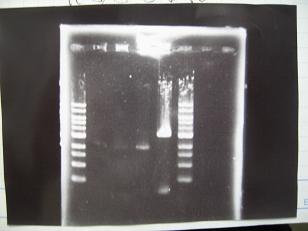
|
|
--->Digestion
(Double Digestion)
- [http://partsregistry.org/Part:BBa_C0178 BBa_C0178]
- [http://partsregistry.org/Part:BBa_C0170 BBa_C0170]
- [http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007)
| Sample No. | 1 | 2 | 3 |
| Sample DNA | 33ng/μl×60μl | 33ng/μl×60μl | 100ng/μl×20μl |
| PstⅠ | 2 | 2 | 2 |
| XbaⅠ | 2 | 2 | - |
| SpeⅠ | - | - | 2 |
| Buffer 2 | - | - | 5 |
| Buffer 3 | 10 | 10 | - |
| BSA | 10 | 10 | 5 |
| dH2O | 16 | 16 | 16 |
| TOTAL | 100 | 100 | 52 |
26 August, 2008
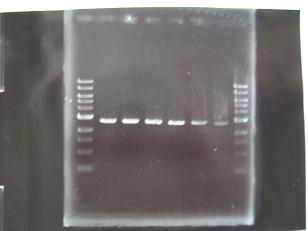
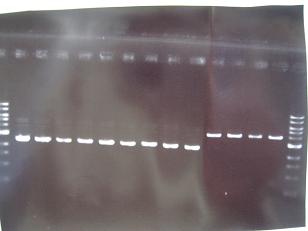
- (8/25)--->Gel Check
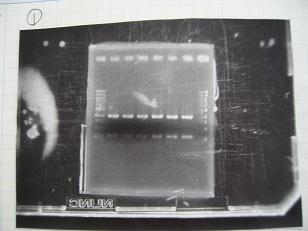
|
|
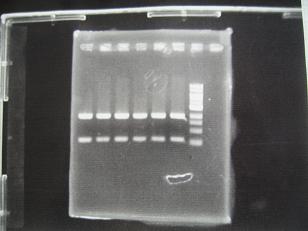
|
|
|
|
--->Gel Extract
- --->Zymo Clean
- 5μl
- 5μl
- 27μl
- --->Gel Check
|
|
- --->SAP
Sample No. 3 Sample DNA 26 SAP 1 SAP Buffer 3 TOTAL 30μL
- --->Zymo Clean
- Sample No.3 -->9μl
- --->Gel Check
Sample No.3 1 Loading Dye 1 dH2O 4 TOTAL 6 - Sample No.3 -> OK ・・・30ng?
- --->Ligation
Sample No. (1) (2) (3) (4) (5) insert①C0178 1 - - 1 - insert②C0170 - 1 - - 1 vector③J04500 - - 2 2 2 ligase 1 1 1 1 1 Buffer 2 2 2 2 2 dH2O 6 6 5 4 4 TOTAL 10 10 10 10 10
- --->Transformation
- insert①C0178
- insert②C0170
- vector③J04500
- insert①C0178+vector③J04500
- insert②C0170+vector③J04500
27 August, 2008
- Sample DNA : BBa_R0010[http://partsregistry.org/Part:BBa_R0010]
Sample No. 1 2 3 Sample DNA 1 1 1 PstⅠ - - 0.5 EcoRⅠ - 0.5 0.5 Buffer 3 - 1 1 BSA - - 1 dH2O - 7.5 2 TOTAL 1 10 10
- --->Gel Check
Sample No. 1 2 3 Sample DNA 1 5 5 Loading Dye 1 1 1 H2O 4 - - TOTAL 6 6 6 - From left;
- BBa_R0010
- Single Digestion
- Double Digestion
- From left;
- (26/8)--->Colony-PCR
- Colony PCR of 5 colonies from ligation plates (26/8-(4),(5)) and one from control plate(BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007)).
DNA Template 1 dNTP mix 5 Foward Primer 5 Reverse Primer 5 DNA polymerase 0.5 Thermopol Buffer 5 dH2O 28.5 TOTAL 50μL
- 95℃,5min -> ( 95℃,1min -> 52℃,1min -> 72℃,1min )・・・25cycles -> 72℃,10min -> 6℃
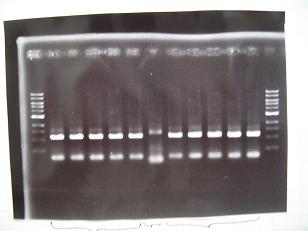
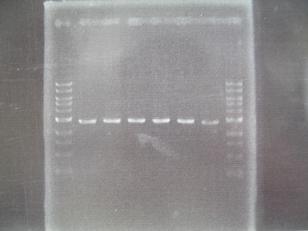
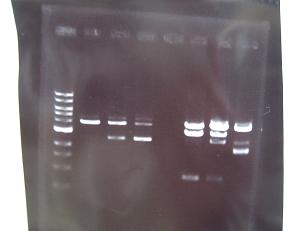
- --->Gel Check
Sample DNA 10 Loading Dye 2 TOTAL 12 - From left;
- 1~5 insert:C0170 + vector:J04500 -> OK
- 6 F2620・・Positive Control -> OK
- 7~11 insert:C0178 + vector:J04500 -> OK
- From left;
- --->(28/8)Miniprep
- competent cells : XL10G
- [http://partsregistry.org/Part:BBa_C0062 BBa_C0062](2007)
- [http://partsregistry.org/Part:BBa_C0062 BBa_C0062](2006) -> no colony on plates
- [http://partsregistry.org/Part:BBa_C0179 BBa_C0179](2007)
- [http://partsregistry.org/Part:BBa_C0179 BBa_C0179](2006)
- [http://partsregistry.org/Part:BBa_S03156 BBa_S03156](2007)
- [http://partsregistry.org/Part:BBa_S03156 BBa_S03156](2006)
- [http://partsregistry.org/Part:BBa_S03158 BBa_S03158](2007)
- [http://partsregistry.org/Part:BBa_S03158 BBa_S03158](2006)
- [http://partsregistry.org/Part:BBa_S03160 BBa_S03160](2007)
- [http://partsregistry.org/Part:BBa_S03160 BBa_S0316](2006)
- --->Mini prep
- --->(29/8)Gel Check
28 August, 2008
- (27/8)--->Mini prep
- insert:C0170 + vector:J04500
- insert:C0178 + vector:J04500
- --->Digestion Test
- insert:C0170 + vector:J04500
- insert:C0178 + vector:J04500
Sample No. 3 4 5 6 insert:C0170 + vector:J04500 1 - 1 - insert:C0178 + vector:J04500 - 1 - 1 EcoRⅠ 0.1 0.1 0.1 0.1 SpeⅠ - - 0.1 0.1 Buffer 3 1 1 1 1 BSA - - 1 1 dH2O 7.8 7.8 6.8 6.8 TOTAL 10 10 10 10
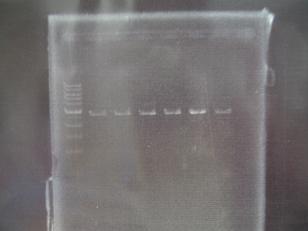
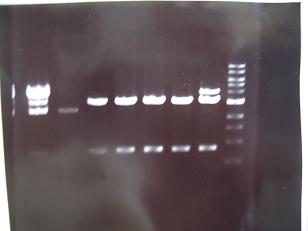
- --->(29/8)Gel Check
Sample No. 1,2 3~6 Sample DNA 1 10 Loading Dye 1 2 H2O 4 - TOTAL 6 12 - From left;
- -> OK
- -> OK
- -> OK
- -> OK
- -> OK
- -> OK
- From left;
29 August, 2008
- --->Digestion Test
- [http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007)
- [http://partsregistry.org/Part:BBa_C0062 BBa_C0062](2007)
- [http://partsregistry.org/Part:BBa_C0179 BBa_C0179](2007)
- [http://partsregistry.org/Part:BBa_C0179 BBa_C0179](2006)
- [http://partsregistry.org/Part:BBa_S03156 BBa_S03156](2007)
- [http://partsregistry.org/Part:BBa_S03156 BBa_S03156](2006)
- [http://partsregistry.org/Part:BBa_S03158 BBa_S03158](2007)
- [http://partsregistry.org/Part:BBa_S03158 BBa_S03158](2006)
- [http://partsregistry.org/Part:BBa_S03160 BBa_S03160](2007)
- [http://partsregistry.org/Part:BBa_S03160 BBa_S03160](2006)
Sample No. S1~10 W1 W2~10 Sample DNA 1 4 2 PstⅠ - 0.075 0.1 EcoRⅠ 0.1 0.075 0.1 Buffer 3 1 0.75 1 BSA - 0.75 1 dH2O 7.9 4.35 5.8 TOTAL 10 10 10
- --->Gel Check
Sample No. 1~10 S1~S10,W1~W10 Sample DNA 1 10 Loading Dye 1 2 TOTAL 6 12 - From left;
- 1~5,S1,S5,W1~W5
- From left;
- From left;
- 6~10,S6~S10,W6~W10
- (27/8)--->Gel Check
Sample DNA 0.5 Loading Dye 1 dH2O 4.5 TOTAL 6μL - R0010 -> OK
- [http://partsregistry.org/Part:BBa_I9026 BBa_I9026](2007)
- [http://partsregistry.org/Part:BBa_I9030 BBa_I9030](2007)
- [http://partsregistry.org/Part:BBa_S03154 BBa_S03154](2007)
- [http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2006)
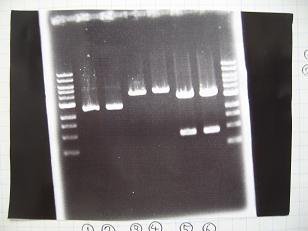
Sample No. 1~3 4 Sample DNA 100ng/μL×20 200ng/μL×30 PstⅠ 2 2 XbaⅠ 2 - SpeⅠ - 2 Buffer 2 - 5 Buffer 3 5 - BSA 5 5 dH2O 16 6 TOTAL 50 50 - --->Gel Check
Sample No. 1~3 4 Sample DNA 10 3 Loading Dye 2 2 dH2O - 7 TOTAL 12 12 - insert-I9026 : 685bp -> None
- insert-I9030 : 745bp -> None
- insert-S03154 : 685bp -> None
- vector-R0010 : 2079bp -> OK
- --->Gel Extract
- --->Zymo Clean
- ->15μL
- --->SAP
Sample No. 4 Sample DNA 15 SAP 2 SAP Buffer 3 dH2O 10 TOTAL 30μL
- --->Zymo Clean
- --->Suspension! (We got information, by the team-output, to the effect that the density of the sample is too low.)
30 August, 2008
- Transformation
- competent cells : XL10G
- [http://partsregistry.org/Part:BBa_I9026 BBa_I9026](2007)
- [http://partsregistry.org/Part:BBa_I9030 BBa_I9030](2006)
- [http://partsregistry.org/Part:BBa_S03154 BBa_S03154](2007)
- [http://partsregistry.org/Part:BBa_S03156 BBa_S03156](2007)
- [http://partsregistry.org/Part:BBa_S03158 BBa_S03158](2007)
- [http://partsregistry.org/Part:BBa_S03160 BBa_S03160](2007)
- [http://partsregistry.org/Part:BBa_C0062 BBa_C0062](2007)
- [http://partsregistry.org/Part:BBa_C0179 BBa_C0179](2007)
- --->(31/8)Mini prep
- (29/8)--->Mini prep
- insert:C0170 + vector:J04500
- insert:C0178 + vector:J04500
- --->Digestion Test
- insert:C0170 + vector:J04500 -> Sample No.1~4
- insert:C0178 + vector:J04500 -> Sample No.5~8
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002](2007) -> Sample No.9
- Single Digestion of Sample No.1~9 -> Sample No.10~18
- Double Digestion of Sample No.1~9 -> Sample No.19~27
Sample No. Single : 10~18 Double : 19~27 Sample DNA 1 5 XbaⅠ 0.1 0.1 SpeⅠ - 0.1 Buffer 2 1 1 BSA 1 1 dH2O 6.9 2.8 TOTAL 10 10
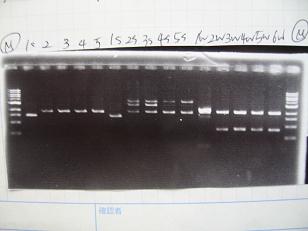
- --->Gel Check
Sample No. 1~9 10~18 19~27 Sample DNA 1 10 10 Loading Dye 1 2 2 dH2O 4 - - TOTAL 6 12 12 - From left;
- Sanple No.1~13
- -> OK
- From left;
- From left;
- Sample No.14~17,24~16
- 14 -> OK
- 15,16 -> Bad
- 17 -> None
- 24~16 -> Bad
- From left;
- Sample No.18,27,19~23
- 18,27 -> Bad
- 19~22 -> OK
- 23 -> OK??
>next week
ホーム メンバー紹介 プロジェクト紹介 Parts Submitted to the Registry モデリング ノート
- --->Zymo Clean
 "
"