Team:UNIPV-Pavia/Notebook/Week15
From 2008.igem.org
(Difference between revisions)
| (7 intermediate revisions not shown) | |||
| Line 46: | Line 46: | ||
!|[[Team:UNIPV-Pavia/Notebook/Week20|Week 20]] | !|[[Team:UNIPV-Pavia/Notebook/Week20|Week 20]] | ||
!|[[Team:UNIPV-Pavia/Notebook/Week21|Week 21]] | !|[[Team:UNIPV-Pavia/Notebook/Week21|Week 21]] | ||
| + | |- | ||
| + | !|[[Team:UNIPV-Pavia/Notebook/Week22|Week 22]] | ||
| + | !|[[Team:UNIPV-Pavia/Notebook/Week23|Week 23]] | ||
| + | !|[[Team:UNIPV-Pavia/Notebook/Week24|Week 24]] | ||
|} | |} | ||
| Line 54: | Line 58: | ||
'''08/25/08''' | '''08/25/08''' | ||
<br> | <br> | ||
| - | * | + | *Plasmid digestion for: |
| - | ** | + | **R0051 (S-P) |
| - | ** | + | **R0040 (S-P) |
| - | **b (E-X) | + | **B0030-C0061-B1006-'''R0062'''-B0030-E0040-B1006 (E-X) (=Lig.b (E-X)) |
| - | ** | + | **B1006 (E-X) |
| - | **12 (E-S) | + | **Lig.12 (E-S) |
*Run/gel extraction. | *Run/gel extraction. | ||
| Line 66: | Line 70: | ||
**BBa_R0051 (S-P) - BBa_E0240 (X-P) (for promoter test) | **BBa_R0051 (S-P) - BBa_E0240 (X-P) (for promoter test) | ||
**BBa_R0040 (S-P) - BBa_E0240 (X-P) (for promoter test) | **BBa_R0040 (S-P) - BBa_E0240 (X-P) (for promoter test) | ||
| - | **12 (E-S) - | + | **Lig.12 (E-S) - B0030-C0061-B1006-'''R0062'''-B0030-E0040-B1006 (E-X) (for AND logic gate test) |
| - | **12 (E-S) - BBa_B1006 (E-X) (to re-perform mutated assemblies) | + | **Lig.12 (E-S) - '''BBa_B1006 (E-X)''' (to re-perform mutated assemblies) |
*We incubated ligations at 16°C overnight. | *We incubated ligations at 16°C overnight. | ||
| + | |||
| + | *We ordered 3OC6HSL (Sigma). | ||
'''08/26/08''' | '''08/26/08''' | ||
| Line 76: | Line 82: | ||
'''08/27/08''' | '''08/27/08''' | ||
<br> | <br> | ||
| - | *Plates grew correctly. We checked colony fluorescence under UV rays: | + | *Plates grew correctly. We checked colony fluorescence of three plates under UV rays: |
| - | ** | + | **'''R0051'''-E0240 glowed |
| - | ** | + | **'''R0040'''-E0240 glowed |
| - | **12-b ( | + | **Lig.12-Lig.b (R0051-B0030-C0062-B0030-C0061-B1006-'''R0062'''-B0030-E0040-B1006) glowed |
| + | |||
| + | *We picked up fluorescent colonies from these three plates to infect three 15 ml falcon tubes containing 1 ml of LB + Amp. We let the culture grow at 37°C, 220 rpm for 3 hours, then we prepared three 50 ul samples and watch them at microscope. Results are shown in The Project section (Experiments). | ||
| + | |||
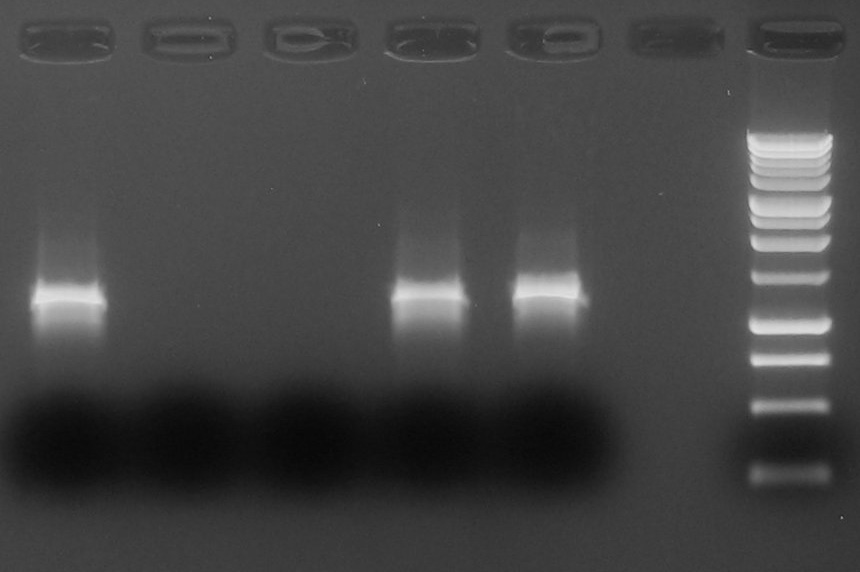
| + | *Colony PCR for 5 colonies of Lig.12-'''B1006''' (called Lig.22). | ||
| + | |||
| + | {| | ||
| + | |[[Image:pv_colonypcr_22again.jpg|thumb|370px|left|Lig.22 (5 colonies), Marker 1Kb]] | ||
| + | |} | ||
| + | |||
| + | *Gel results: OK! we chose 1st colony to grow a 9 ml overnight culture. | ||
| + | |||
| + | '''08/28/08''' | ||
| + | <br> | ||
| + | *Glycerol stocks/miniprep for Lig.22. | ||
| + | |||
| + | *We sent purified plasmids to Primm for sequencing. | ||
| + | |||
| + | *We infected 9 ml of LB + Amp with 30 µl of R0062 glycerol stock. | ||
| + | |||
| + | '''08/29/08''' | ||
| + | <br> | ||
| + | *Glycerol stock/miniprep for R0062. | ||
Latest revision as of 21:27, 26 October 2008
Notebook
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
|---|---|---|---|---|---|---|
| Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 |
| Week 15 | Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | Week 21 |
| Week 22 | Week 23 | Week 24 |
Week 15: 08/25/08 - 08/29/08
08/25/08
- Plasmid digestion for:
- R0051 (S-P)
- R0040 (S-P)
- B0030-C0061-B1006-R0062-B0030-E0040-B1006 (E-X) (=Lig.b (E-X))
- B1006 (E-X)
- Lig.12 (E-S)
- Run/gel extraction.
- Ligations:
- BBa_R0051 (S-P) - BBa_E0240 (X-P) (for promoter test)
- BBa_R0040 (S-P) - BBa_E0240 (X-P) (for promoter test)
- Lig.12 (E-S) - B0030-C0061-B1006-R0062-B0030-E0040-B1006 (E-X) (for AND logic gate test)
- Lig.12 (E-S) - BBa_B1006 (E-X) (to re-perform mutated assemblies)
- We incubated ligations at 16°C overnight.
- We ordered 3OC6HSL (Sigma).
08/26/08
- We transformed/plated ligations.
08/27/08
- Plates grew correctly. We checked colony fluorescence of three plates under UV rays:
- R0051-E0240 glowed
- R0040-E0240 glowed
- Lig.12-Lig.b (R0051-B0030-C0062-B0030-C0061-B1006-R0062-B0030-E0040-B1006) glowed
- We picked up fluorescent colonies from these three plates to infect three 15 ml falcon tubes containing 1 ml of LB + Amp. We let the culture grow at 37°C, 220 rpm for 3 hours, then we prepared three 50 ul samples and watch them at microscope. Results are shown in The Project section (Experiments).
- Colony PCR for 5 colonies of Lig.12-B1006 (called Lig.22).
- Gel results: OK! we chose 1st colony to grow a 9 ml overnight culture.
08/28/08
- Glycerol stocks/miniprep for Lig.22.
- We sent purified plasmids to Primm for sequencing.
- We infected 9 ml of LB + Amp with 30 µl of R0062 glycerol stock.
08/29/08
- Glycerol stock/miniprep for R0062.
 "
"