Team:Chiba/jk/β/week 3
From 2008.igem.org
(Difference between revisions)
m (→3 September, 2008) |
m (→5 September, 2008) |
||
| Line 738: | Line 738: | ||
:Cultured the following cells (2mL LB-Amp, at 37℃) | :Cultured the following cells (2mL LB-Amp, at 37℃) | ||
::from transformed plates: | ::from transformed plates: | ||
| - | ::*BBa_K084009(Plac+RBS+RhlI+LVA, Competent Cells : | + | ::*BBa_K084009(Plac+RBS+RhlI+LVA, Competent Cells : JW1908) [http://partsregistry.org/Part:BBa_K084009](r1, r3~r7) |
| - | ::*BBa_K084010(Plac+RBS+RhlI, Competent Cells : | + | ::*BBa_K084010(Plac+RBS+RhlI, Competent Cells : JW1908)[http://partsregistry.org/Part:BBa_K084010](c1,c2,c4~c8) |
::from Glycerol Stock: | ::from Glycerol Stock: | ||
| - | ::*BBa_S03623(Ptet+RBS+LuxI, Competent Cells : | + | ::*BBa_S03623(Ptet+RBS+LuxI, Competent Cells : JW1908)[http://partsregistry.org/Part:BBa_S03623] |
:--->'''Phenotype test''' | :--->'''Phenotype test''' | ||
| Line 770: | Line 770: | ||
*Incubated for 8hours at 37 degrees | *Incubated for 8hours at 37 degrees | ||
| - | *The measurement of the intensity GFP by | + | *The measurement of the intensity GFP by Fluoroskan Ascent 2.5(program:Ascent Software Version 2.6) |
<table width="500" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | <table width="500" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
<tr> | <tr> | ||
Revision as of 12:15, 30 September 2008
>送受信班
Contents |
Week 3
31 August, 2008
- Transformation
- Competent Cells : XL10G
- BBa_K084007(Plac+RBS+LasI)[http://partsregistry.org/Part:BBa_K084007]
- BBa_K084008(Plac+RBS+RhlI)[http://partsregistry.org/Part:BBa_K084008]
- ---> We saved these plates with a refrigerator.
- Digestion
- BBa_I9026[http://partsregistry.org/Part:BBa_I9026](2007)
- BBa_I9030[http://partsregistry.org/Part:BBa_I9030](2006)
- BBa_S03154[http://partsregistry.org/Part:BBa_S03154](2007)
- BBa_R0010[http://partsregistry.org/Part:BBa_R0010](2007)
Sample No 1~3 4 Sample DNA 12 10 PstⅠ 0.2 0.2 XbaⅠ 0.2 - SpeⅠ - 0.4 Buffer 2 - 1.5 Buffer 3 2 - BSA 2 1.5 dH2O 3.6 1.4 TOTAL 20 15
- --->(1/9)Gel Check
- (30/8)--->Mini prep
- --->Digestion test
- Plac+RBS+RhlI Sample No.2~5
- BBa_T9002[http://partsregistry.org/Part:BBa_T9002]①、②
Sample Single Digesiton 2~5 Double Digestion 2~5 Single Digestion T9002① Single Digesiton T9002② Double Digestion T9002①② Sample DNA 1 3 3 1 3 XbaⅠ 0.1 0.1 0.1 0.1 0.1 SpeⅠ - 0.1 - - 0.1 Buffer 2 0.9 0.8 0.9 0.9 0.8 BSA 1 1 1 1 1 dH2O 7 5 5 7 5 TOTAL 10 10 10 10 10
- --->Gel Check
1 September, 2008
- (31/8)--->Gel Check
Sample No 1~3 4 Sample DNA 20 15 Loading Dye 4 3 TOTAL 24 18 - From left;
- insert-1(I9026)
- insert-2(I9030)
- From left;
- insert-3(S03154)
- vector-4(R0010)
- --->Gel extract
- --->zymo
- insert-1(I9026) -> 7μL
- insert-2(I9030) -> 7μL
- insert-3(S03154) -> 7μL
- vector-4(R0010) -> 15μL
- --->SAP
- vector-4(R0010)
- --->Zymo
- vector-4(R0010) -> 20μL
- --->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- insert-1(I9026) -> OK
- insert-2(I9030) -> OK
- insert-3(S03154) -> None --> Transformation BBa_S03154[http://partsregistry.org/Part:BBa_S03154]-->
(2/9)Mini prepwith BBa_K084009[http://partsregistry.org/Part:BBa_K084009], BBa_K084010[http://partsregistry.org/Part:BBa_K084010]
- vector-4(R0010)
- From left;
- --->Ligation
Sample No (1) (2) (3) (4) (5) insert-1(I9026) 3 - 3 - - insert-2(I9030) - 3 - 3 - vector-4(R0010) 3 3 - - 3 ligase 1 1 1 1 1 Buffer 1 1 1 1 1 dH2O 2 2 5 5 5 TOTAL 10 10 10 10 10
- --->Transformation
- Competent cells : XL10GOLD 30μL
- Transformed the following and grew on new ampicillin plates.
- BBa_K084009(Plac+RBS+RhlI+LVA, Amp)[http://partsregistry.org/Part:BBa_K084009] -> 628 colonies
- BBa_K084010(Plac+RBS+CinI+LVA, Amp)[http://partsregistry.org/Part:BBa_K084010] -> 500 colonies
- insert-1(RBS+RhlI+LVA) -> 9 colonies
- insert-2(RBS+CinI+LVA) -> No colonies on the plate
- vector-4(Plac, Amp) -> 186 colonies
- --->(2/9) Colony PCR
- Competent cells : BW ΔFliC 40μL
- Transformed the following and grew on new ampicillin plates.
- BBa_K084007(Plac+RBS+LasI)[http://partsregistry.org/Part:BBa_K084007]
- BBa_K084008(Plac+RBS+RhlI)[http://partsregistry.org/Part:BBa_K084008]
- BBa_T9002(Ptet+RBS+LuxR+GFP)[http://partsregistry.org/Part:BBa_K084010]
- --->(2/9)Liquid Culture
2 September, 2008
- (31/8)--->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- I9026 -> OK, 100/μL
- I9030 -> OK, 50ng/μL
- S03154 -> OK, 30ng/μL (too low for the ligation:1/9 )
- From left;
- (1/9)---> Colony PCR
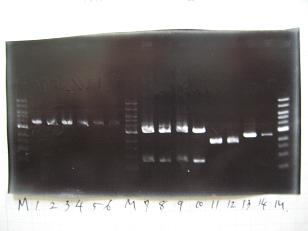
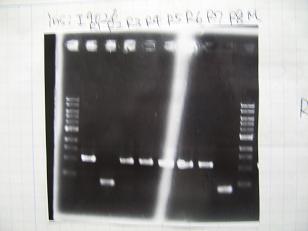
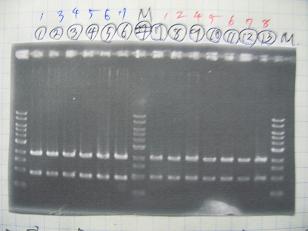
- Colony PCR of 8 colonies from ligation plates (1/9:(1)BBa_K084009[http://partsregistry.org/Part:BBa_K084009](R1~R8),(2)BBa_K084010[http://partsregistry.org/Part:BBa_K084010](C1~C8)) and one from control plate(BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007)).
DNA Template 1 dNTP mix 5 Foward Primer 0.3 Reverse Primer 0.3 DNA polymerase TAQ 0.5 Thermopol Buffer 3 dH2O 20.5 TOTAL 30μL
- 95℃,5min -> ( 95℃,1min -> 52℃,1min -> 72℃,1min )・・・25cycles -> 72℃,10min -> 6℃
--->Gel CheckSample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- Plac+RBS+RhlI+LVA
- R1 -> OK
- R2 -> Bad
- R3~R7 -> OK
- R8 -> Bad
- From left;
- From left;
- Plac+RBS+CinI+LVA
- C1,C2 -> OK
- C3 -> Bad
- C4~C6 -> OK
- From left;
- Plac+RBS+CinI+LVA
- C7,C8 -> OK
- BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007):Positive control -> OK
- --->(3/9)Mini prep
(1/9)--->Liquid Culture- Cultured the following cells (2mL LB-Amp, at 37℃, 7 hours)
- from transformed plates:
- BBa_K084007(Plac+RBS+LasI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084007]
- BBa_K084008(Plac+RBS+RhlI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084008]
- BBa_T9002(Ptet+RBS+LuxR+GFP, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_T9002]
- from Glycerol Stock:
- BBa_S03623(Ptet+RBS+LuxI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_S03623]
- from transformed plates:
--->(3/9)Phenotype test
- Competent cells : XL10G 30μL
- C0161(2007) [http://partsregistry.org/Part:BBa_C0161]
- C0161(2006) [http://partsregistry.org/Part:BBa_C0161]
- C0261(2007) [http://partsregistry.org/Part:BBa_C0261]
- C0261(2006) [http://partsregistry.org/Part:BBa_C0261]
--->(4/9)Mini prep
3 September, 2008
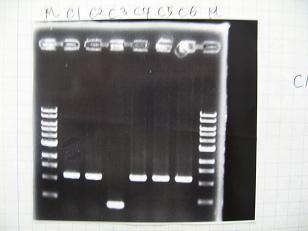
- (2/9)--->Mini prep
- BBa_K084009[http://partsregistry.org/Part:BBa_K084009](R1, R3~R7)
- BBa_K084010[http://partsregistry.org/Part:BBa_K084010](C1,C2,C4~C8)
- BBa_S03154[http://partsregistry.org/Part:BBa_S03154]
- --->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- S03154[http://partsregistry.org/Part:BBa_S03154] -> OK 33ng/μL
- BBa_K084009[http://partsregistry.org/Part:BBa_K084009](R1, R3~R7) -> OK
- From left;
- From left;
- BBa_K084010[http://partsregistry.org/Part:BBa_K084010](C1,C2,C4~C8) -> OK
- --->Digestion test
- BBa_K084009[http://partsregistry.org/Part:BBa_K084009](R1, R3~R7)
- BBa_K084010[http://partsregistry.org/Part:BBa_K084010](C1,C2,C4~C8)
Digestion Single Double Sample DNA 1 3 XbaⅠ 0.1 0.1 PstⅠ 0.1 0.1 Buffer 2 0.9 - Buffer 3 - -0.8 BSA 1 1 dH2O 7 5 TOTAL 10μL 10μL
- --->Gel Check
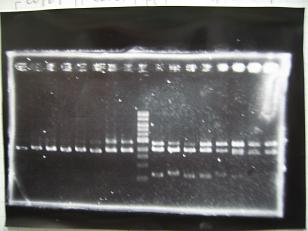
Sample DNA 10 Loading Dye 2 TOTAL 12 - From left;
- Single Digestion : BBa_K084009[http://partsregistry.org/Part:BBa_K084009]R1,R3~R7 -> OK
- Single Digestion : BBa_K084010[http://partsregistry.org/Part:BBa_K084010]C1,C2,C4~C8 -> OK
- From left;
- From left;
- Double Digestion : BBa_K084009[http://partsregistry.org/Part:BBa_K084009]R1,R3~R7 -> OK
- Double Digestion : BBa_K084010[http://partsregistry.org/Part:BBa_K084010]C1,C2,C4~C8 -> OK
- (2/9)--->Phenotype-test
- MIX
- BBa_K084007(Plac+RBS+LasI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084007] -> Sample Name : L1~L4
- BBa_K084008(Plac+RBS+RhlI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084008] -> Sample Name : R1~R4
- BBa_S03623(Ptet+RBS+LuxI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_S03623]
- BBa_T9002(Ptet+RBS+LuxR+GFP, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_T9002]
Sample No. 1 2 3 4 5 6 7 8 9 10 11 L1 2mL - - - - - - - - - - L2 - 2mL - - - - - - - - - L3 - - 2mL - - - - - - - - L4 - - - 2mL - - - - - - - R1 - - - - 1mL - - - - - - R2 - - - - - 1mL - - - - - R3 - - - - - - 1mL - - - - R4 - - - - - - - 1mL - - - BBa_S03154[http://partsregistry.org/Part:BBa_S03154] - - - - - - - - 2mL - - AHL(100μM) - - - - - - - - - - 1μL BBa_T9002[http://partsregistry.org/Part:BBa_T9002] 2mL 2mL 2mL 2mL 1mL 1mL 1mL 1mL 2mL 1mL 1mL IPTG(100μM) 1μL 1μL 1μL 1μL 1μL 1μL 1μL 1μL - - - - Incubated for 8hours at 37 degrees
- Spindown (max rpm, 3 min)
- The measurement of the intensity GFP(visual judgment)
Sample No 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP + + + + + + + ++ + +++ -
- Leaving for 12 at room temperture.
- Resuspension
- The measurement of the intensity GFP by KEIKOU-READER
Sample No 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP 25.65 18.91 20.39 21.85 26.62 26.58 30.07 33.60 27.41 10.72 55.54 4 September, 2008
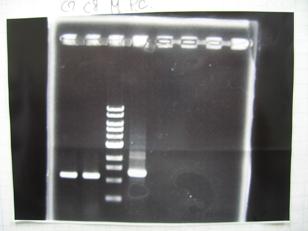
- --->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6μL - From left;
- BBa_C0161(2007)[http://partsregistry.org/Part:BBa_C0161] -> OK
- BBa_C0161(2007)[http://partsregistry.org/Part:BBa_C0161] -> OK
- BBa_C0161(2006)[http://partsregistry.org/Part:BBa_C0161] -> OK
- BBa_C0161(2006)[http://partsregistry.org/Part:BBa_C0161] -> OK
- From left;
- From left;
- BBa_C0261(2007)[http://partsregistry.org/Part:BBa_C0161] -> OK
- BBa_C0261(2007)[http://partsregistry.org/Part:BBa_C0161] -> OK
- BBa_C0261(2006)[http://partsregistry.org/Part:BBa_C0161] -> OK
- BBa_C0261(2006)[http://partsregistry.org/Part:BBa_C0161] -> OK
- --->Digestion test
Sample Single Digesiton Double Digestion Sample DNA 1 2 EcoRⅠ 0.05 0.05 PstⅠ - 0.05 Buffer 3 1 1 BSA 1 1 dH2O 6.95 5.9 TOTAL 10μL 10μL
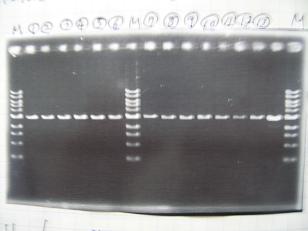
- --->Gel Check
Sample DNA 10 Loading Dye 2 TOTAL 12μL
- From left;
- Single Digestion
- BBa_C0161(2007)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0161(2007)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0161(2006)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0161(2006)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0261(2007)[http://partsregistry.org/Part:BBa_C0261]
- BBa_C0261(2007)[http://partsregistry.org/Part:BBa_C0261]
- BBa_C0261(2006)[http://partsregistry.org/Part:BBa_C0261]
- BBa_C0261(2006)[http://partsregistry.org/Part:BBa_C0261]
- ladder
- Double digestion
- BBa_C0161(2007)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0161(2007)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0161(2006)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0161(2006)[http://partsregistry.org/Part:BBa_C0161]
- BBa_C0261(2007)[http://partsregistry.org/Part:BBa_C0261]
- BBa_C0261(2007)[http://partsregistry.org/Part:BBa_C0261]
- BBa_C0261(2006)[http://partsregistry.org/Part:BBa_C0261]
- BBa_C0261(2006)[http://partsregistry.org/Part:BBa_C0261]
- Single Digestion
- Transformation
- Competent cells : BW ΔFliC
- Transformed the following and grew on new ampicillin plates.
- BBa_K084009[http://partsregistry.org/Part:BBa_K084009](R-3,4,6,7)
- BBa_K084010[http://partsregistry.org/Part:BBa_K084010](C-1,2,6,7)
- BBa_T9002(2007)[http://partsregistry.org/Part:BBa_T9002]
- --->(5/9)Liquid Culture
5 September, 2008
- (4/9)--->Liquid Culture
- Cultured the following cells (2mL LB-Amp, at 37℃)
- from transformed plates:
- BBa_K084009(Plac+RBS+RhlI+LVA, Competent Cells : JW1908) [http://partsregistry.org/Part:BBa_K084009](r1, r3~r7)
- BBa_K084010(Plac+RBS+RhlI, Competent Cells : JW1908)[http://partsregistry.org/Part:BBa_K084010](c1,c2,c4~c8)
- from Glycerol Stock:
- BBa_S03623(Ptet+RBS+LuxI, Competent Cells : JW1908)[http://partsregistry.org/Part:BBa_S03623]
- from transformed plates:
- --->Phenotype test
- MIX
Sample No. 1 2 3 4 5 6 7 8 9 10 11 r3 1mL - - - - - - - - - - r4 - 1mL - - - - - - - - - r6 - - 1mL - - - - - - - - r7 - - - 1mL - - - - - - - c1 - - - - 1mL - - - - - - c2 - - - - - 1mL - - - - - c6 - - - - - - 1mL - - - - c7 - - - - - - - 1mL - - - BBa_S03154[http://partsregistry.org/Part:BBa_S03154] - - - - - - - - 1mL - - AHL(100μM) - - - - - - - - - - 1μL BBa_T9002[http://partsregistry.org/Part:BBa_T9002] 1mL 1mL 1mL 1mL 1mL 1mL 1mL 1mL 1mL 1mL 1mL IPTG(100μM) 1μL 1μL 1μL 1μL 1μL 1μL 1μL 1μL - - -
- Incubated for 8hours at 37 degrees
- The measurement of the intensity GFP by Fluoroskan Ascent 2.5(program:Ascent Software Version 2.6)
Sample No 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP (Frist run) 15.21 16.44 16.34 16.63 9.720 9.703 9.685 9.874 19.95 16.59 71.04 the intensity GFP (Second run) 17.85 19.19 19.28 19.14 9.981 10.16 10.14 10.41 23.94 17.61 79.53
- Spindown (max rpm, 3 min)
- The measurement of the intensity GFP(visual judgment) (UV 312nm)
Sample No 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP + + + + - - - - + ++ - 6 September, 2008
- PCR of BBa_I9030 and two control,BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007)).
DNA Template 1 dNTP mix 5 Foward Primer 5 Reverse Primer 5 DNA polymerase 1 Thermopol Buffer 5 dH2O 33 TOTAL 50μL
- 95℃,5min -> ( 95℃,1min -> 54℃,1min -> 72℃,1min )・・・25cycles -> 72℃,10min -> 6℃ store
- --->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
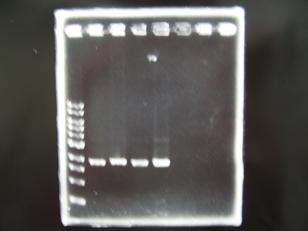
- 1 I9030 without LVA -> OK
- 2 I9030 Positive Control -> OK
- 3 F2620 Positive control -> OK
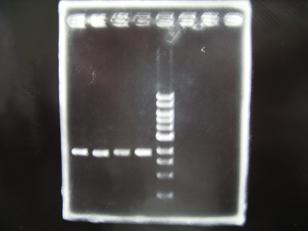
Gel Check Sample DNA : R0010
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6
- Sample DNA:
1:R0010,2:S03154,3:J04500,4:C0161,5:C0261
Sample No 1 2 3 4 5 Sample DNA 1 1 1 1 1 PstⅠ 0.5 0.5 0.5 0.5 0.5 SpeⅠ 0.5 - 1 - - XbaⅠ - 0.5 - 0.5 0.5 Buffer 2 2 - 3 - - Buffer 3 - 6 - 6 6 (10×)BSA 2 0.6 3 - - (100×)BSA - - - 0.6 0.6 dH2O 3 6.4 7 1.4 1.4 TOTAL 20 60 30 60 60 >next week
ホーム メンバー紹介 プロジェクト紹介 Parts Submitted to the Registry モデリング ノート
- From left;
 "
"