Team:Chiba/jk/β/week 3
From 2008.igem.org
(Difference between revisions)
(→2 September, 2008) |
|||
| Line 264: | Line 264: | ||
| - | :--->(2/9)''' | + | :--->(2/9)'''[[Team:Chiba/protocol/PCR| Colony PCR]]''' |
| Line 275: | Line 275: | ||
::*BBa_T9002(Ptet+RBS+LuxR+GFP)[http://partsregistry.org/Part:BBa_K084010] | ::*BBa_T9002(Ptet+RBS+LuxR+GFP)[http://partsregistry.org/Part:BBa_K084010] | ||
| - | :--->(2/9)'''Liquid Culture | + | :--->(2/9)'''Liquid Culture''' |
===2 September, 2008=== | ===2 September, 2008=== | ||
| Line 306: | Line 306: | ||
| - | :(1/9)--->'''[[Team:Chiba/protocol/PCR|Colony | + | :(1/9)--->'''[[Team:Chiba/protocol/PCR| Colony PCR]]''' |
::Colony PCR of 8 colonies from ligation plates (1/9-(1),(2)) and one from control plate(BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007)). | ::Colony PCR of 8 colonies from ligation plates (1/9-(1),(2)) and one from control plate(BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007)). | ||
| Line 403: | Line 403: | ||
| - | :(1/9)---> | + | :(1/9)--->'''Liquid Culture''' |
| + | :Cultured the following cells (2mL LB-Amp, at 37℃, 7 hours) | ||
| + | ::from transformed plates: | ||
| + | ::*BBa_K084007(Plac+RBS+LasI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084007] | ||
| + | ::*BBa_K084008(Plac+RBS+RhlI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084008] | ||
| + | ::*BBa_T9002(Ptet+RBS+LuxR+GFP, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084010] | ||
| + | ::from Glycerol Stock: | ||
| + | ::*BBa_S03623(Ptet+RBS+LuxI, Competent Cells : BW ΔFliC) | ||
| + | |||
| + | :--->(3/9)'''Phenotype test''' | ||
| Line 527: | Line 536: | ||
| - | (2/9)--->'''Phenotype-test | + | :(2/9)--->'''Phenotype-test''' |
| + | :*MIX | ||
:<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | :<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
<tr> | <tr> | ||
| Line 545: | Line 555: | ||
<tr><td>R3</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>1</td><td>-</td><td>-</td><td>-</td><td>-</td></tr> | <tr><td>R3</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>1</td><td>-</td><td>-</td><td>-</td><td>-</td></tr> | ||
<tr><td>R4</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>1</td><td>-</td><td>-</td><td>-</td></tr> | <tr><td>R4</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>1</td><td>-</td><td>-</td><td>-</td></tr> | ||
| - | |||
<tr><td>BBa_S03154[http://partsregistry.org/Part:BBa_S03154]</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>2</td><td>-</td><td>-</td></tr> | <tr><td>BBa_S03154[http://partsregistry.org/Part:BBa_S03154]</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>-</td><td>2</td><td>-</td><td>-</td></tr> | ||
<tr><td>BBa_T9002[http://partsregistry.org/Part:BBa_T9002]</td><td>2</td><td>2</td><td>2</td><td>2</td><td>1</td><td>1</td><td>1</td><td>1</td><td>2</td><td>1</td><td>1</td></tr> | <tr><td>BBa_T9002[http://partsregistry.org/Part:BBa_T9002]</td><td>2</td><td>2</td><td>2</td><td>2</td><td>1</td><td>1</td><td>1</td><td>1</td><td>2</td><td>1</td><td>1</td></tr> | ||
| - | <tr><td>AHL</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td></tr> | + | <tr><td>AHL(100μM)</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td><td>1</td></tr> |
</table> | </table> | ||
| - | + | ||
| - | : | + | :*Incubated for 8hours at 37 degrees |
| - | : | + | :*Spindown (max rpm, 3 min) |
| - | + | :*The measurement of the intensity GFP by Masahiro Tominaga | |
| - | : | + | ::<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> |
| - | :: | + | <tr> |
| - | + | <td width="257">Sample No</td> | |
| - | + | <td>1</td><td>2</td><td>3</td><td>4</td><td>5</td><td>6</td><td>7</td><td>8</td><td>9</td><td>10</td><td>11</td> | |
| + | </tr> | ||
| + | <tr> | ||
| + | <td>the intensity GFP</td> | ||
| + | <td>+</td><td>+</td><td>+</td><td>+</td><td>+</td><td>+</td><td>+</td><td>++</td><td>+</td><td>-</td><td>+++</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |||
| + | |||
===4 September, 2008=== | ===4 September, 2008=== | ||
Revision as of 12:05, 26 September 2008
>送受信班
Contents |
Week 3
31 August, 2008
- Transformation
- Competent Cells : XL10G
- BBa_K084007(Plac+RBS+LasI)[http://partsregistry.org/Part:BBa_K084007]
- BBa_K084008(Plac+RBS+RhlI)[http://partsregistry.org/Part:BBa_K084008]
- ---> We saved these plates with a refrigerator.
- Digestion
- BBa_I9026[http://partsregistry.org/Part:BBa_I9026](2007)
- BBa_I9030[http://partsregistry.org/Part:BBa_I9030](2006)
- BBa_S03154[http://partsregistry.org/Part:BBa_S03154](2007)
- BBa_R0010[http://partsregistry.org/Part:BBa_R0010](2007)
Sample No 1~3 4 Sample DNA 12 10 PstⅠ 0.2 0.2 XbaⅠ 0.2 - SpeⅠ - 0.4 Buffer 2 - 1.5 Buffer 3 2 - BSA 2 1.5 dH2O 3.6 1.4 TOTAL 20 15
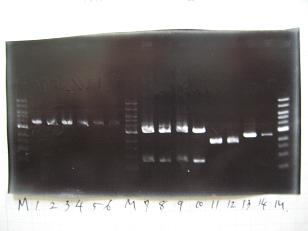
- --->(1/9)Gel Check
- (30/8)--->Mini prep
- --->Digestion test
- Plac+RBS+RhlI Sample No.2~5
- BBa_T9002[http://partsregistry.org/Part:BBa_T9002]①、②
Sample Single Digesiton 2~5 Double Digestion 2~5 Single Digestion T9002① Single Digesiton T9002② Double Digestion T9002①② Sample DNA 1 3 3 1 3 XbaⅠ 0.1 0.1 0.1 0.1 0.1 SpeⅠ - 0.1 - - 0.1 Buffer 2 0.9 0.8 0.9 0.9 0.8 BSA 1 1 1 1 1 dH2O 7 5 5 7 5 TOTAL 10 10 10 10 10
- --->Gel Check
1 September, 2008
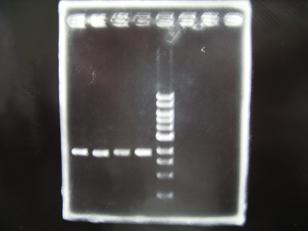
- (31/8)--->Gel Check
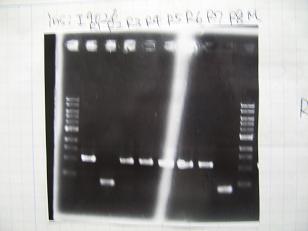
Sample No 1~3 4 Sample DNA 20 15 Loading Dye 4 3 TOTAL 24 18 - From left;
- insert-1(I9026)
- insert-2(I9030)
- From left;
- insert-3(S03154)
- vector-4(R0010)
- --->Gel extract
- --->zymo
- insert-1(I9026) -> 7μL
- insert-2(I9030) -> 7μL
- insert-3(S03154) -> 7μL
- vector-4(R0010) -> 15μL
- --->SAP
- vector-4(R0010)
- --->Zymo
- vector-4(R0010) -> 20μL
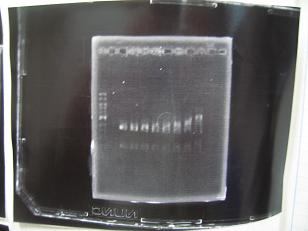
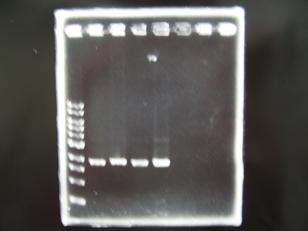
- --->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- insert-1(I9026) -> OK
- insert-2(I9030) -> OK
- insert-3(S03154) -> None --> Transformation BBa_S03154[http://partsregistry.org/Part:BBa_S03154]
- vector-4(R0010) -> OK
- From left;
- --->Ligation
Sample No (1) (2) (3) (4) (5) insert-1(I9026) 3 - 3 - - insert-2(I9030) - 3 - 3 - vector-4(R0010) 3 3 - - 3 ligase 1 1 1 1 1 Buffer 1 1 1 1 1 dH2O 2 2 5 5 5 TOTAL 10 10 10 10 10
- --->Transformation
- Competent cells : XL10GOLD 30μL
- Transformed the following and grew on new ampicillin plates.
- BBa_K084009(Plac+RBS+RhlI+LVA, Amp)[http://partsregistry.org/Part:BBa_K084009] -> 628 colonies
- BBa_K084010(Plac+RBS+CinI+LVA, Amp)[http://partsregistry.org/Part:BBa_K084010] -> 500 colonies
- insert-1(RBS+RhlI+LVA) -> 9 colonies
- insert-2(RBS+CinI+LVA) -> No colonies on the plate
- vector-4(Plac, Amp) -> 186 colonies
- --->(2/9) Colony PCR
- --->Transformation
- Competent cells : BW ΔFliC 40μL
- Transformed the following and grew on new ampicillin plates.
- BBa_K084007(Plac+RBS+LasI)[http://partsregistry.org/Part:BBa_K084007]
- BBa_K084008(Plac+RBS+RhlI)[http://partsregistry.org/Part:BBa_K084008]
- BBa_T9002(Ptet+RBS+LuxR+GFP)[http://partsregistry.org/Part:BBa_K084010]
- --->(2/9)Liquid Culture
2 September, 2008
- (31/8)--->Gel Check
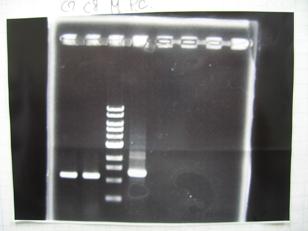
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- I9026 -> OK, 100/μL
- I9030 -> OK, 50ng/μL
- S03154 -> OK, 30ng/μL (too low for the ligation:1/9 )
- From left;
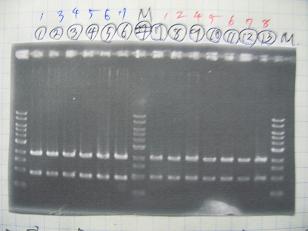
- (1/9)---> Colony PCR
- Colony PCR of 8 colonies from ligation plates (1/9-(1),(2)) and one from control plate(BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007)).
DNA Template 1 dNTP mix 5 Foward Primer 0.3 Reverse Primer 0.3 DNA polymerase TAQ 0.5 Thermopol Buffer 3 dH2O 20.5 TOTAL 30μL
- 95℃,5min -> ( 95℃,1min -> 52℃,1min -> 72℃,1min )・・・25cycles -> 72℃,10min -> 6℃
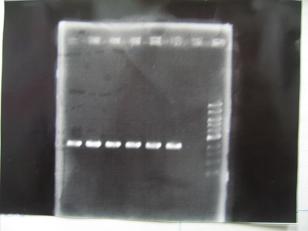
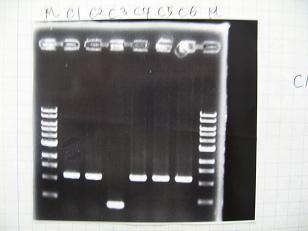
--->Gel CheckSample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- Plac+RBS+RhlI+LVA
- R1 -> OK
- R2 -> Bad
- R3~R7 -> OK
- R8 -> Bad
- From left;
- From left;
- Plac+RBS+CinI+LVA
- C1,C2 -> OK
- C3 -> Bad
- C4~C6 -> OK
- From left;
- Plac+RBS+CinI+LVA
- C7,C8 -> OK
- BBa_F2620[http://partsregistry.org/Part:BBa_F2620](2007):Positive control -> OK
- --->(3/9)Mini prep
- (1/9)--->Liquid Culture
- Cultured the following cells (2mL LB-Amp, at 37℃, 7 hours)
- from transformed plates:
- BBa_K084007(Plac+RBS+LasI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084007]
- BBa_K084008(Plac+RBS+RhlI, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084008]
- BBa_T9002(Ptet+RBS+LuxR+GFP, Competent Cells : BW ΔFliC)[http://partsregistry.org/Part:BBa_K084010]
- from Glycerol Stock:
- BBa_S03623(Ptet+RBS+LuxI, Competent Cells : BW ΔFliC)
- from transformed plates:
- --->(3/9)Phenotype test
- Competent cells : XL10G 30μL
- C0161(2007) [http://partsregistry.org/Part:BBa_C0161]
- C0161(2006) [http://partsregistry.org/Part:BBa_C0161]
- C0261(2007) [http://partsregistry.org/Part:BBa_C0261]
- C0261(2006) [http://partsregistry.org/Part:BBa_C0261]
- --->(4/9)Mini prep
3 September, 2008
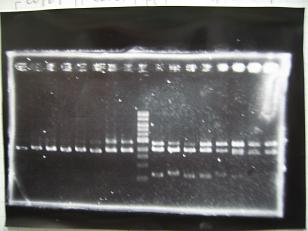
- (2/9)--->Mini prep
- --->Gel Check
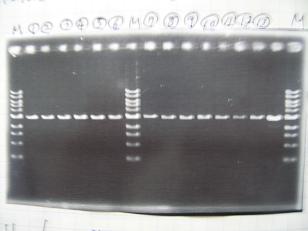
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- S03154 -> OK
- R1,R3~R7 (Plac+RBS+RhlI+LVA) -> OK
- From left;
- From left;
- C1,C2,C4~C8 (Plac+RBS+CinI+LVA) -> OK
- --->Digestion test
- R1,R3~R7 (Plac+RBS+RhlI+LVA)
- C1,C2,C4~C8 (Plac+RBS+CinI+LVA)
Digestion Single Double Sample DNA 1 3 XbaⅠ 0.1 0.1 PstⅠ 0.1 0.1 Buffer 2 0.9 - Buffer 3 - -0.8 BSA 1 1 dH2O 7 5 TOTAL 10 10
- --->Gel Check
Sample DNA 10 Loading Dye 2 TOTAL 12 - From left;
- Single Digestion : R1,R3~R7 -> OK
- Single Digestion : C1,C2,C4~C8 -> OK
- From left;
- From left;
- Double Digestion : R1,R3~R7 -> OK
- Double Digestion : C1,C2,C4~C8 -> OK
- (2/9)--->Phenotype-test
- MIX
Sample No. 1 2 3 4 5 6 7 8 9 10 11 L1 2 - - - - - - - - - - L2 - 2 - - - - - - - - - L3 - - 2 - - - - - - - - L4 - - - 2 - - - - - - - R1 - - - - 1 - - - - - - R2 - - - - - 1 - - - - - R3 - - - - - - 1 - - - - R4 - - - - - - - 1 - - - BBa_S03154[http://partsregistry.org/Part:BBa_S03154] - - - - - - - - 2 - - BBa_T9002[http://partsregistry.org/Part:BBa_T9002] 2 2 2 2 1 1 1 1 2 1 1 AHL(100μM) 1 1 1 1 1 1 1 1 1 1 1 - Incubated for 8hours at 37 degrees
- Spindown (max rpm, 3 min)
- The measurement of the intensity GFP by Masahiro Tominaga
Sample No 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP + + + + + + + ++ + - +++
4 September, 2008
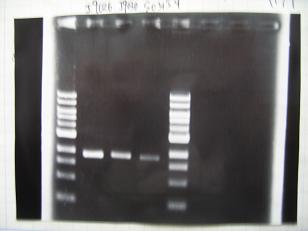
- --->Gel Check
Sample No ① ② ③ ④ Sample DNA 3 3 3 3 Loading Dye 2 2 2 2 dH2O 7 7 7 7 TOTAL 12 12 12 12 - From left;
Sample No ① ② ③ ④ Sample DNA 3 3 3 3 Loading Dye 2 2 2 2 dH2O 7 7 7 7 TOTAL 12 12 12 12 - From left;
Sample No ① ② ③ ④ Sample DNA 3 3 3 3 Loading Dye 2 2 2 2 dH2O 7 7 7 7 TOTAL 12 12 12 12 - From left;
5 September, 2008
6 September, 2008
>next week
ホーム メンバー紹介 プロジェクト紹介 Parts Submitted to the Registry モデリング ノート
 "
"