Team:Bologna/Wetlab
From 2008.igem.org
(→Uv Sensitive Trigger) |
(→Uv Sensitive Trigger) |
||
| Line 33: | Line 33: | ||
BBa_K079049 and BBa_K079050 are two new constructs submitted to the registry by Bologna’s Igem Team 2008. These UV test circuits can be presented schematically by the following sequences with different promoter (J23118 with 1429 strength and J23100 with 2547 strength). | BBa_K079049 and BBa_K079050 are two new constructs submitted to the registry by Bologna’s Igem Team 2008. These UV test circuits can be presented schematically by the following sequences with different promoter (J23118 with 1429 strength and J23100 with 2547 strength). | ||
| - | [[Image:fig050.jpg|center|thumbnail|Figure 1: BBa_K079050]] | + | [[Image:fig050.jpg|center|thumbnail|500 px |Figure 1: BBa_K079050]] |
[[Image:fig049.jpg|center|thumbnail|Figure 2: BBa_K079049]] | [[Image:fig049.jpg|center|thumbnail|Figure 2: BBa_K079049]] | ||
<br> | <br> | ||
Revision as of 16:19, 29 October 2008
| HOME | PROJECT | TEAM | SOFTWARE | MODELING | WET LAB | LAB-BOOK | SUBMITTED PARTS | BIOSAFETY AND PROTOCOLS |
|---|
Contents |
LAB Experiment
Uv Sensitive Trigger
In the genetic flip-flop, the amount of LacI to set on the memory is induced by an UV sensitive trigger. Production of LacI molecules by UV induction can be tested replacing the LacI gene by GFP, so it is possible to have a relation of the strength of LacI synthesis measuring the value of fluorescence. BBa_K079049 and BBa_K079050 are two new constructs submitted to the registry by Bologna’s Igem Team 2008. These UV test circuits can be presented schematically by the following sequences with different promoter (J23118 with 1429 strength and J23100 with 2547 strength).
To test the UV induction we used the UV illuminator.
UV Induction
Plasmids with the constructs have been transformed in DH5alfa bacteria by standard protocol and one colony from the plate was picked up and cultured overnight in 5ml LB medium broth with ampicillin. The day after the culture was diluted in 5ml LB and antibiotic in order to have OD = 0.1 and let it grown for another one hour; after that the culture was divided in five 15ml tubes (1ml of bacteria per tube), in order to be induced. Using 1ml of culture is a choice done in order to have a thickness as thick as possible to perform an irradiation as uniform as possible .
Tests with difference distances from the lamp with different exposition times were done to respect the maximum lethal UV dose of bacteria and avoid mutagenesis factors in the E. coli bacteria cells. The following table illustrates the test setup used and OD after one hour:
After induction by UV the samples were kept for 2 hours in dark by silver paper to increase the RecA and LexA response. The OD samples was measured and the sample transferred in a 1ml tube, spinned at 6000-8000rpm for three minutes and the supernatant was discard and the pellet resuspended for the slide preparation in order to acquire and elaborate the bacterial fluorescence view by microscope and Bacteria Visual Fluo Software.
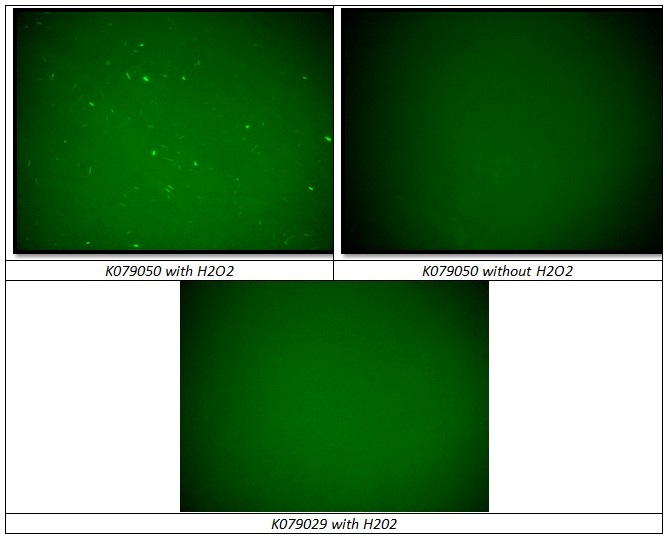
Unfortunately, using 15ml tube and maybe the not the correct time/distance induction, the experimental tests didn’t shown GFP expression. It could be depended because of not uniform irradiation of the sample. In the Fig. is possible to see a picture of leak answer by bacteria stimulated by UV at the distance of 4cm using less than one second time as exposure.
Hydrogen Peroxide Induction
The reaction of hydrogen peroxide with transition metals imposes on cells an oxidative stress condition that can result in damage to cell components such as proteins, lipids and principally to DNA. Escherichia Coli cells are able to deal with these adverse events via DNA repair mechanisms or OxyR and SosRS anti-oxidant inducible pathways which are elicited by cells to avoid the introduction of oxidative lesions by hydrogen peroxide. Among the systems the OxyR gene interacts with, there is the SOS response. Since we have not success with UV induction, to test the correct functionality of K079049 and K079050 constructs, they were induced by peroxide hydrogen. Low concentration of (1-3mM) results in SOS gene induction in wild-type cells [Imlay and Linn, 1987; Goerlich et alt. 1989].
The plasmid contained the LexA Operator was transformed into DH5alfa bacteria according to the standard protocol and one colony was picked up from the plate and let it grown overnight in 15ml tube with 5ml LB broth and ampicillin. Cultured colony was diluted in 5ml LB broth with ampicillin and 1ul H2O2 (11M) to have medium with 2.2mM concentration of H202 and 0.1 starting OD. Testing BBa_K079050 construct, two negative controls were used to prove that H202 induction works correctly and that can be used in flip-flop without interferences between Lac Operon and SOS System:
- BBa_K079050 grown in LB medium without H202
- BBa_K079029 grown in LB medium with H202 (Fig. )
Tubes were kept to the dark by using silver paper and let them growing for 2 hours at 37°C. 1 ml of bacteria sample has been collected and transferred inside 1ml tube and spinned at 6000-8000 rpm for 3 minutes. The supernatant was thrown away and the pellet resuspended for the slide preparation.
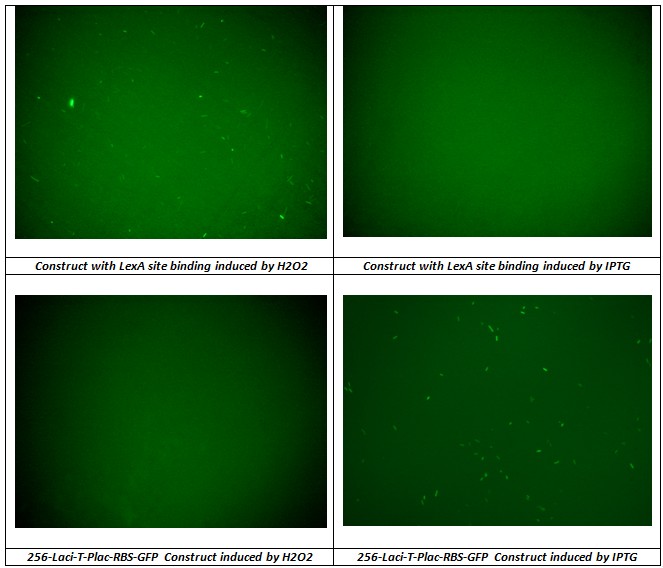
In addition to prove that K079050 construct can be used in the flip-flop circuit without interference between Lac Operon and SOS System a test IPTG and H202 of two previously constructs were done. Fig. shows the results.
As expected the construct with Lex A operator induced by IPTG and Lac-Operon-based-circuit induced by H2O2 didn’t produced GFP synthesis, instead the other two sample correctly induced presented good level of fluorescence.
We conclude that H2O2 induction gives an uniform activity of the regulator promoter (J23100) and the level of promoter activity can be used to set on the memory.
Homemade UV Illuminator
The UV source that we use is a GW6 Sylvania, a lamp that emitt UVC at 253,7nm near the absorption's peak of DNA with an optical output power of 1,6W; to use it safely we built a box of mdf(medium density-fibreboard) that surrounds the lamp and prevent the UVc leakeage, moreover we use a diaphragm that allows passage only to the light absorbing the remainder. We insert in that box two pierced brackets, that permits to choose the distance between the lamp and the sample and then in accord with the Lambert-Beer law's the exposure time. Finally we embedded this structure in a polistyrene box for its handling and greater safety (Figure 2-3).
Gel matrix
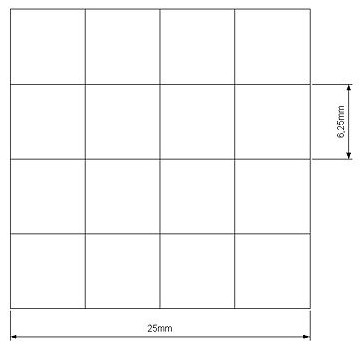
Our project use UV light for its space selectivity, that gives the possibility to irradiate a target zone without interfering with the other. To do that we build a mold to make a matrix of agarose gel;this give us a square of 25mm side's with 16 cells inside where we can locate bacteria( Figure 4 )
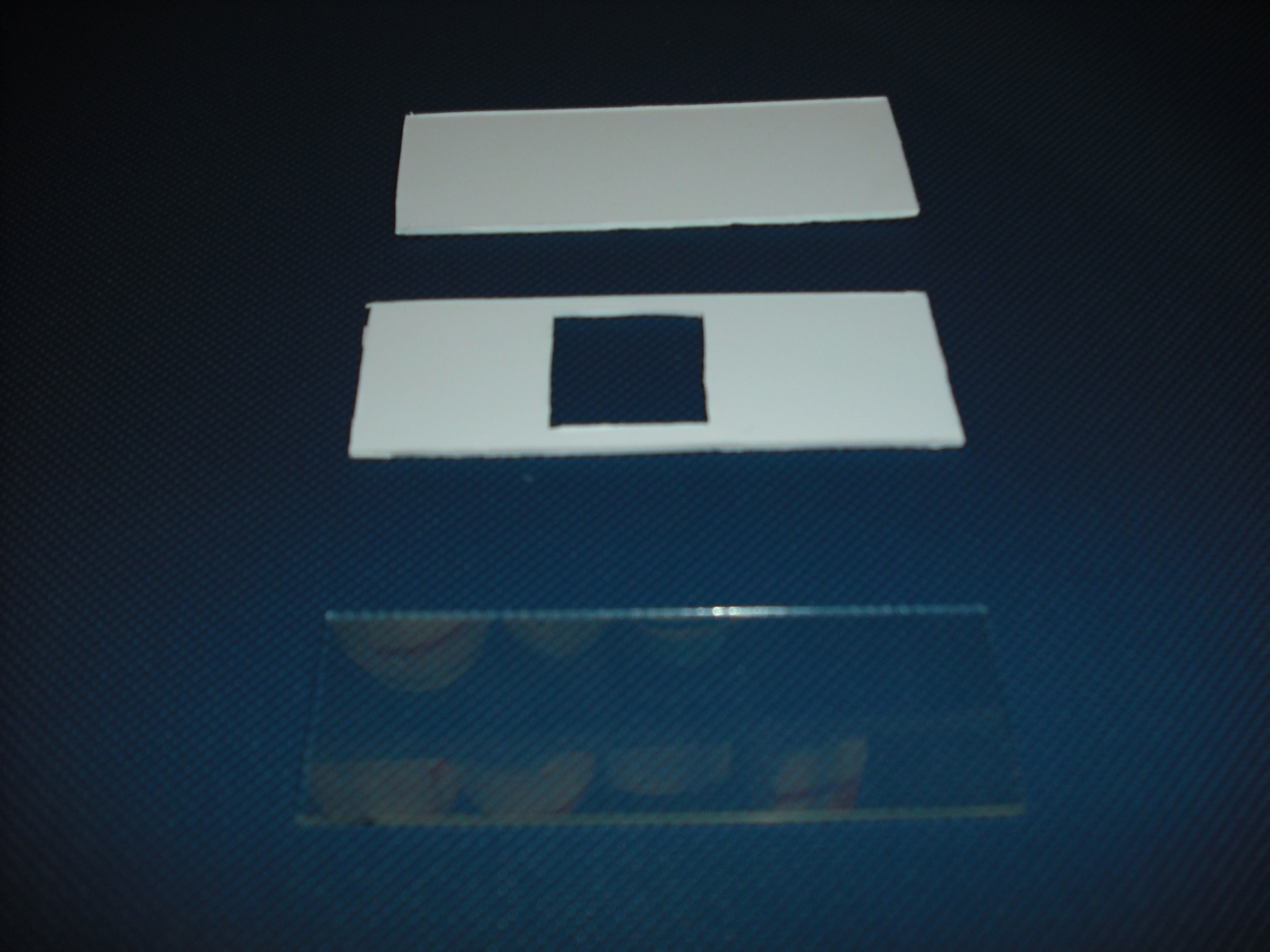
Once do that is easy with an optical mask, like those used in photolithography for electronics
circuits, stimulate only the selected bacteria.To realize this device we use palsticard because it is
very easy to shape and clean( Figure 5 ).
The mold is made of two parts that will form a sandwich with the slide: one will create the shape
of the gel matrix the other is a cover that that will give the picture into gel .
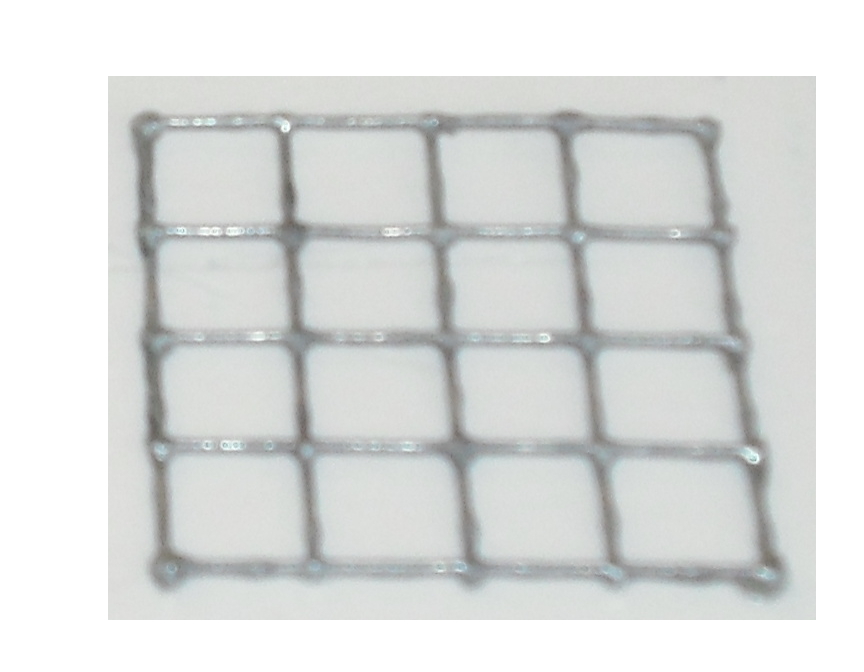
The matrix is obtained through the pressure of the cover part over the mold part
and that is the result:
Operator sites are dna sequences very small in length (15 to 20 bp) and a restriction digestion for the religation in standard plasmids is not possible with the existing purification kits. In fact, only Dna sequences down to at least 40 bp can be purified with the standard kits available. So, we decided to set a new protocol up for the isolation and cloning of single operator sites into standard BioBricks plasmids.
We started from the analysis of an existing promoter library into the Registry (link). As it can be seen in figure, these plasmids were meant for the construction of promoter basic parts and their derivatives. They can be used two ways: 1. Insertion of a promoter element between XbaI and SpeI sites results in a RFP reporter while retaining the ability to do BioBrick assembly. Part J61002 is the tet promoter variant of the plasmid. 2. Insertion of a protein generating device or RNA gene (cutting the part with XbaI/PstI, inserting into SpeI/PstI of J61002) results in a standard pSB1A2 plasmid containing an r0040.yourpart composite.
FOTO!!!!
Thus, we decided to isolate the RFP protein from one of the promoter family member with a SpeI- PstI enzymatic digestion. Then, this part could be assemble with an operator sequence digested SpeI- PstI, too. This could allow the isolation of the Operator- RFP sequence from the commercial plasmid and the subsequent religation into a standard plasmid. Then, a final extraction of the RFP gene with a SpeI- PstI digestion would leave the operator site inside the standard plasmid.
 "
"