Team:TUDelft/Temperature design2
From 2008.igem.org
>> Work in progress
Contents |
Parts Design II
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K115016 BBa:K115016], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115017 BBa:K115017], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115018 BBa:K115018], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115019 BBa:K115019]
Changing the temperature threshold
The principle of the RNA thermometer is based on base pairing between the nucleotides in the Shine Dalgarno region. At a certain temperature the RNA is folded into a structure in which base pairing nucleotides make the Shine Dalgarno region unreachable for the ribosome. With a rise in temperature, the binding forces between the base pairing nucleotides decrease and above a certain threshold temperature the binding forces between the base pairs are to weak to hold the base pairing nucleotides together, causing the RNA to (partially) unfold. The binding between the base pairing nucleotides let loose, exposing the Shine-Dalgarno region and thereby enabling the ribosome to initiate translation.
When only looking at this principle, an RNA thermometer with a different temperature threshold can be designed by simply increasing or decreasing the binding forces of the base-pairing nucleotides in the Shine-Dalgarno region, shifting the temperature threshold to a higher or lower temperature respectively (fig). Increasing the binding forces could be achieved by incorporating base pairing C and G nucleotides, which bind relatively strong forming a stable helix. Decreasing the binding forces could be done by introducing less strong binding nucleotide base-pairs, such as A-U and G-U base pairs, forming a less stable helix.
But things are not that easy. At first, adding mutations in order to enforce or weaken the temperature sensitive region can also cause the RNA to fold into a completely different structure. This way it can lose its function as an RNA thermometer. Secondly, there are some constraints to the possible mutations; the start codon and the Shine Dalgarno sequence must off course remain unaltered and in case of a standard biobrick the scar must also be part of the temperature sensitive region (see the scar problem). Third, although it seems that only a thermosensitive hairpin is needed to have a functioning RNA thermometer (as described in the analysis section) there could very well be more factors that are of influence on the functioning and temperature threshold of the RNA thermometer that are still unknown.
Design approach
Starting point
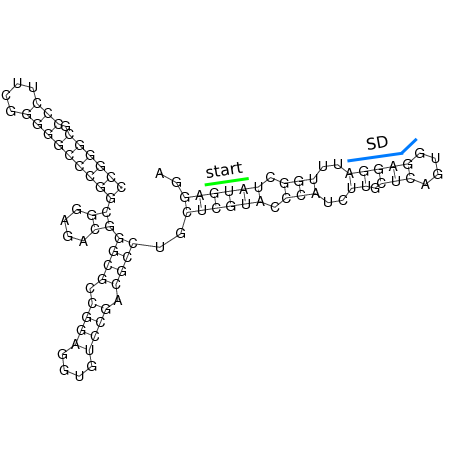
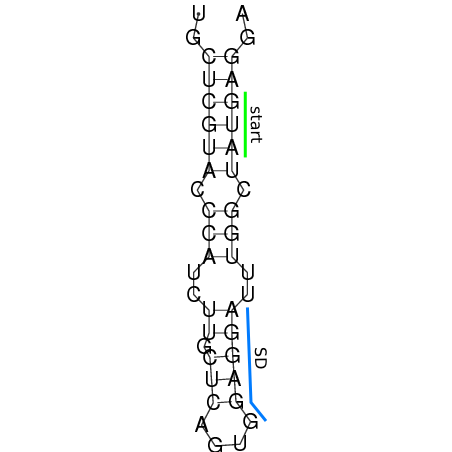
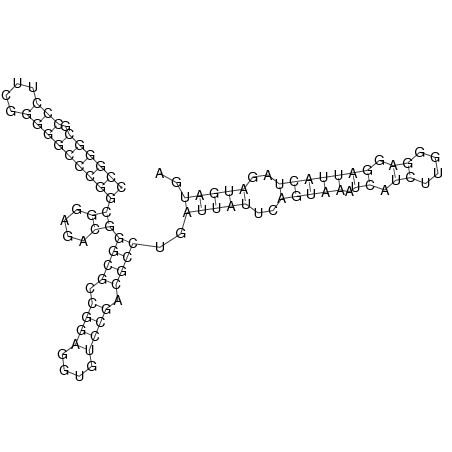
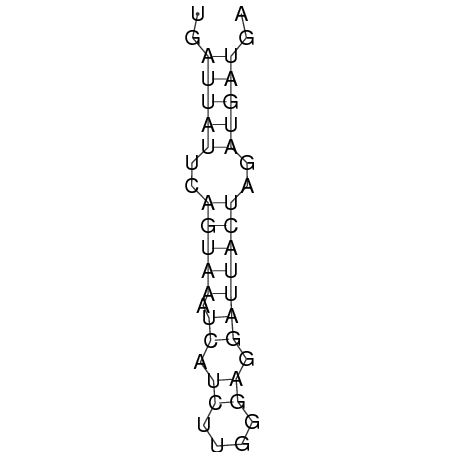
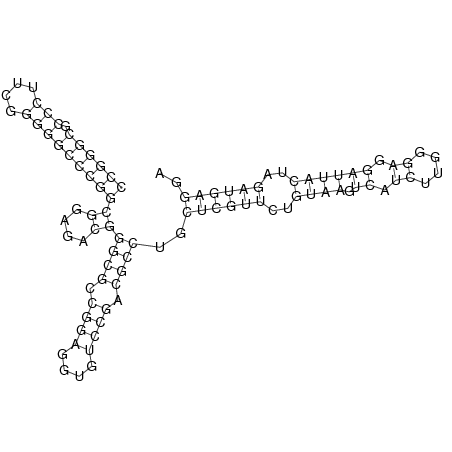
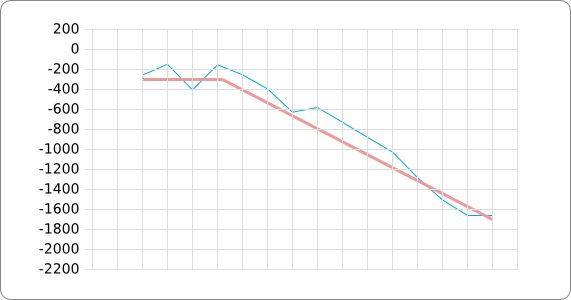
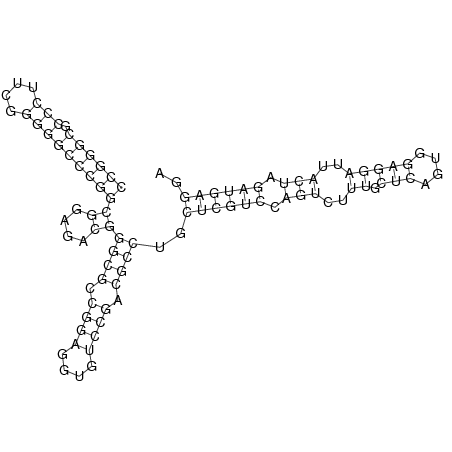
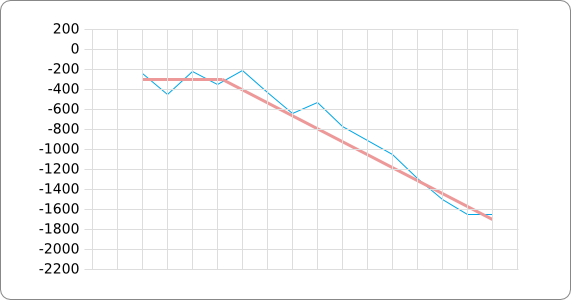
In order to design an RNA thermometer with a different temperature threshold an existing RNA thermometer is taken as a starting point (TODO: explain). The one chosen is a ROSE RNA thermometer from Bradyrhizobium japonicum USDA 110 ([http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&val=NC_004463 NC_004463]) residing at location [http://www.ncbi.nlm.nih.gov/projects/sviewer/?id=NC_004463.1&v=5784144-5784239 5784144-5784239] within the genome which is at the 5' side of the gene [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1051455&ordinalpos=2&itool=EntrezSystem2.PEntrez.Gene.Gene_ResultsPanel.Gene_RVDocSum hspB]. The reason that a sequence of the ROSE family is chosen instead of one of the FourU family is that there are more ROSE than FourU sequences available which can be used for the analysis of the temperature sensitive part of the RNA thermometer. The reason that a sequence of the ROSE family is chosen instead of one of the PrfA family is that there are more papers that provide information about the working and effects of mutation on ROSE RNA thermometers than on PrfA RNA thermometers. There is no specific reason for the choice of this ROSE RNA thermometer, this is just a ROSE RNA thermometers taken from the [http://rfam.sanger.ac.uk/family?entry=rose Rfam database]. The figure below shows the secondary structure of the whole RNA thermometer and the temperature sensitive hairpin as predicted by [http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi RNAfold] together with a plot of the stacking energies compared to the stacking energies trend as found in the analysis (TODO: link).
Design requirements
Having a starting point we need a way to turn this RNA thermometer that functions at 37 degrees Celcius into an RNA thermometer that functions at a different temperature and that can be turned into a standard biobrick. It is not possible to just introduce mutations anywhere in order to, for example, make the temperature sensitive hairpin less stable which should theoretically lower the temperature threshold. There are certain restriction to the possible mutation in that there must be a start codon, a Shine Dalgarno sequence, and a scar present. Also the order and distances between these parts are predefined.
Some other requirements are stated in order to simplify the design process and making it easier to predict expected temperature threshold. At first the sequence that precedes the thermosensitive hairpin is fixed. This is done because it is still unknown what the function and influence on the temperature threshold of these extra hairpins is. Altering this sequence would thus not be usefull because we cannot tell what the concequenses of these alterations would be. As a second requirement it is stated that the overal structure of the temperature sensitive structure should remain the same. This means that the length of the hairpin is fixed, as well as the location and the size of the loop at the end of the hairpin. This requirement is also stated in order to simplify the design process. This restriction greatly reduces the solution space compared to for example the situation where there is no restriction to the length of the hairpin. It also enables to make a better and more confident prediction of the functioning of the RNA thermometer. The analysis (reference or link) showed that mutations influence the translation efficiency but that the function as RNA thermometer can remain. It is unclear if the same would hold when for example the length of the temperature sensitive hairpin is changed.
Recapulating, the list of requirements is now as follows:
- The sequence before the temperature sensitive hairpin remains unaltered, so mutations are only allowed within the temperature sensitive hairpin.
- The overall structure of the temperature sensitive hairpin must remain the same. This means that the loop at the end should be of the same size and at the same position and the length of the hairpin remains the same. There are no restrictions to the number and locations of internal loops and bulges within the temperature sensitive hairpin.
- The start codon, SD sequence, and scar sequence are predefined sequences and are placed at fixed location within the structure.
These requirements are captured in figure x that can be seen as a template for the design of the temperature sensitive hairpin.
Design heuristic
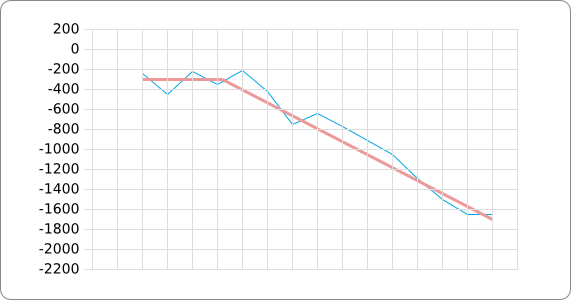
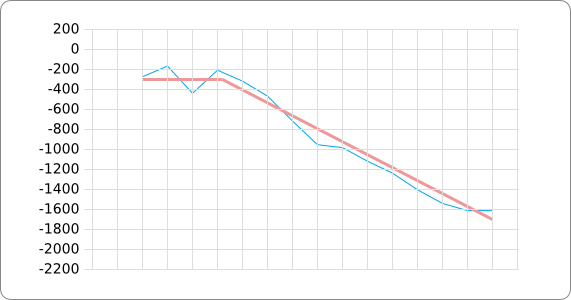
The analysis of the temperature sensitive (TODO: link) hairpin of the known ROSE RNA thermometers has shown a trend in the stacking energies of the base pairs that is common to the different thermometers (figure). This trend observed for the stacking energies calculated at 37 degrees Celsius, the situation where the ROSE RNA thermometer is switched on (figure). As a convention we will call this a 37 degrees Celsius switch.
To design a switch with a different temperature switching point, for example a 27 degrees Celsius switch, we assume that the stacking energies plot of this RNA thermometer, calculated at 27 degrees, should be similar to the trend we found for the 37 degrees Celsius switch (calculated at 37 degrees). This would mean that the stability of the temperature sensitive hairpin of the 27 degrees Celsius switch at 27 degrees Celsius is the same as the stability of the temperature sensitive hairpin of the 37 degrees Celsius switch at 37 degrees.
A design can now be made by fitting the stacking energies plot (calculated at the desired temperature) of the designed hairpin to the found stacking energies trend, taking into account the mentioned requirements in the previous section.
The design of a temperature sensitive hairpin that 'switches' at a certain temperature can be made using the following heuristic:
- Choose a desired switching temperature.
- Take a sequence with the predefined nucleotides (start codon, SD seqeunce, and the scar) present at the correct locations, the rest of the nucleotides can be filled at random.
- Predict the secondary structure of the RNA thermometer (at the desired switching temperature) to check if it corresponds to the required structure. If not, go to step 2.
- Compare the stacking energies plot (calculated for the desired switching temperature) of the temperature sensitive hairpin to the trend plot and calculate the distance between the two plots. If it is the smallest distance thus far, remember the sequence as the best one thus far and go back to step 2 to give it another try. Otherwise go back to step 2.
In order to break this loop a conditional check can be added to the last step that ensures that the algorithm is stopped when the found distance is small enough, i.e. the fit is good enough.
Note This heuristic is purely illustrative, only showing the basic principle. When put into an algorithm, a different approach is needed to make it an efficient algorithm.
It takes to much time to implement such an algorithm within the time frame of this project. It is also not yet shown that this design method produces working RNA thermometers, so it would be to early to spend a lot of time on the design of such an algorithm.
Design of a 27°C, 32°C, and 37°C switch
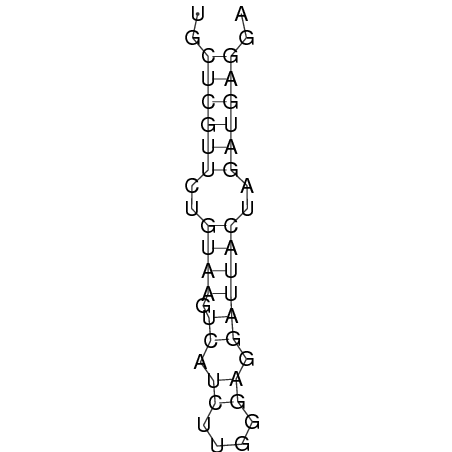
The design heuristic, as proposed in the previous section, is used to design three RNA thermometers, a 27°C, a 32°C, and a 37°C version (these are the temperatures at which the RNA thermometers are theoretically switched on). As also mentioned in the previous section, there is no algorithm to perform the optimization process, so the designs are made manually. [RNAfold] is used to predict the secondary structure at a certain temperature in the second step and [RNAeval] is used to calculate the stacking energies at a certain temperature in the third step. Fitting the plot is done visually by comparing the energy stacking plot of the design to the trend line as in the rightmost figures below, so there is no calculation method used to determine the distance between these two graphs. The termination of the design process is thus also not well defined. The sequence was altered until the stacking energy plot fitted the trend comparably as the existing 37°C RNA thermometers do. The resulting RNA thermometers are displayed below together with the plot of the stacking energies compared to the trend line.
Temperature sensitive hairpin parts
It is shown that an RNA thermometer also functions when it only consists of the temperature sensitive hairpin (ref Chowdhurry). The results of the experiment indicate that the temperature threshold shifts upward in case of the temperature sensitive hairpin only version. To test this, the designed parts are also added as a temperature sensitive hairpin version only.
If these short hairpins happen to be enough to get a working RNA thermometer, this would be a nice starting point for a third design phase in which these small parts are further optimized. For us, there's not enough time left to do this, but it could be a good starting point for a next years iGEM project.
References
- ^ De Smit M H, Van Duijn J (1990). "Secondary structure of the ribosome binding site determines translation efficiency: A quantitative analysis". PNAS, 1990-10, vol.87, no.19, 7668-7672. [http://www.ncbi.nlm.nih.gov/pubmed/2217199 PMID:2217199]
- ^ Hoe N P, Goguen J D (1993). "Temperature sensing in Yersinia pestis: Translation of the LcrF activator protein is thermally regulated". J Bacteriol, 1993 December, 175(24), 7901-7909. [http://www.ncbi.nlm.nih.gov/pubmed/7504666 PMID:7504666]
- ^ Chowdhurry S, Maris C, Allain F H T, Narberhaus F (2006). "Molecular basis for temperature sensing by an RNA thermometer". The EMBO Journal, 2006, 25, 2487–2497. [http://www.ncbi.nlm.nih.gov/pubmed/16710302 PMID:16710302]
- ^ Nocker A, Hausherr T, Balsiger S, Krstulovic N, Hennecke H, Narberhaus F (2001). "A mRNA-based thermosensor controls expression of rhizobial heat shock genes". Nucleic Acids Research, 2001 December 1, 29(23):4800-4807. [http://www.ncbi.nlm.nih.gov/pubmed/11726689 PMID:11726689]
- ^ Balsiger S, Ragaz C, Baron C, Narberhaus F. "Replicon-specific regulation of small heat shock genes in Agrobacterium tumefaciens". Journal of Bacteriology, October 2004, p. 6824-6829, Vol.186, No.20. [http://www.ncbi.nlm.nih.gov/pubmed/15466035 PMID:15466035]
- ^ Waldminghaus T, Heidrich N, Branti S, Narberhaus F (2007). "FourU: a novel type of RNA thermometer in Salmonella". Molecular Microbiology, Volume 65, Issue 2, 413-424. [http://www.ncbi.nlm.nih.gov/pubmed/17630972 PMID:17630972]
- ^ Johansson J, Mandin P, Renzoni A, Chiaruttinni C, Springer M, Cossart P. "An RNA thermosensor controls expression of virulance genes in Listeria monocytogenes". Cell , Volume 110 , Issue 5 , 551-561. [http://www.ncbi.nlm.nih.gov/pubmed/12230973 PMID:12230973]
- ^ Chowdhury S, Ragaz C, Kreuger E, and Narberhaus F (2003). "Temperature-controlled Structural Alterations of an RNA Thermometer". J. Biol. Chem.,November 28, 2003, Vol. 278, Issue 48, 47915-47921. [http://www.ncbi.nlm.nih.gov/pubmed/12963744 PMID:12963744]
 "
"