Team:Paris/July 28
From 2008.igem.org
(→Results of the extraction : Electrophoresis) |
|||
| (27 intermediate revisions not shown) | |||
| Line 406: | Line 406: | ||
! rowspan="1"| 6 | ! rowspan="1"| 6 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: #ccccff;"| | + | ! rowspan="2" style="background: #ccccff;"|D125 |
| - | ! rowspan="2" style="background: #ccccff;"| | + | ! rowspan="2" style="background: #ccccff;"|B0015 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"|EcoRI | ! rowspan="2"|EcoRI | ||
| Line 532: | Line 532: | ||
| - | ==> Conclusion : for most of the samples we have enough signal to determine the concentration of the digestion to do the ligation. | + | ==> ''Conclusion :'' for most of the samples we have enough signal to determine the concentration of the digestion to do the ligation. |
| Line 538: | Line 538: | ||
| - | ==> Conclusion : The gel n°7 allowed us to know the concentration of other digestion undetermined. | + | ==> ''Conclusion :'' The gel n°7 allowed us to know the concentration of other digestion undetermined. |
| - | + | ||
| - | + | ||
| - | + | ||
== Ligation == | == Ligation == | ||
| Line 555: | Line 552: | ||
* 2 µl Ligase Buffer 10x | * 2 µl Ligase Buffer 10x | ||
* 20 µl qsp H2O | * 20 µl qsp H2O | ||
| - | |||
* Incubates O/N at 4°C (in the fridge) | * Incubates O/N at 4°C (in the fridge) | ||
| + | === List of ligations === | ||
{| border="1" | {| border="1" | ||
| Line 578: | Line 575: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L101 |
|align=center| D110 | |align=center| D110 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 586: | Line 583: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L102 |
|align=center| D129 | |align=center| D129 | ||
| - | |align=center| | + | |align=center| D118 |
|align=center| A | |align=center| A | ||
|align=center|5 | |align=center|5 | ||
| Line 594: | Line 591: | ||
|align=center|2 | |align=center|2 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L103 |
|align=center| D129 | |align=center| D129 | ||
|align=center| D122 | |align=center| D122 | ||
| Line 602: | Line 599: | ||
|align=center|7 | |align=center|7 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L104 |
|align=center| D129 | |align=center| D129 | ||
|align=center| D114 | |align=center| D114 | ||
| Line 610: | Line 607: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L105 |
|align=center| D123 | |align=center| D123 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 618: | Line 615: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L106 |
|align=center| D123 | |align=center| D123 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 626: | Line 623: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L107 |
|align=center| D103 | |align=center| D103 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 634: | Line 631: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L108 |
|align=center| D103 | |align=center| D103 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 642: | Line 639: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L109 |
|align=center| D124 | |align=center| D124 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 650: | Line 647: | ||
|align=center|6 | |align=center|6 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L110 |
|align=center| D124 | |align=center| D124 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 658: | Line 655: | ||
|align=center|6 | |align=center|6 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L111 |
|align=center| D104 | |align=center| D104 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 666: | Line 663: | ||
|align=center|9 | |align=center|9 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L112 |
|align=center| D104 | |align=center| D104 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 674: | Line 671: | ||
|align=center|9 | |align=center|9 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L113 |
|align=center| D126 | |align=center| D126 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 682: | Line 679: | ||
|align=center|9.5 | |align=center|9.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L114 |
|align=center| D126 | |align=center| D126 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 690: | Line 687: | ||
|align=center|9.5 | |align=center|9.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L115 |
|align=center| D105 | |align=center| D105 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 698: | Line 695: | ||
|align=center|- | |align=center|- | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L116 |
|align=center| D105 | |align=center| D105 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 706: | Line 703: | ||
|align=center|- | |align=center|- | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L117 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D117 | |align=center| D117 | ||
| Line 714: | Line 711: | ||
|align=center|10 | |align=center|10 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L118 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D121 | |align=center| D121 | ||
| Line 722: | Line 719: | ||
|align=center|11 | |align=center|11 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L119 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D113 | |align=center| D113 | ||
| Line 730: | Line 727: | ||
|align=center|11 | |align=center|11 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L120 |
|align=center| D106 | |align=center| D106 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 738: | Line 735: | ||
|align=center|3.5 | |align=center|3.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L121 |
|align=center| D106 | |align=center| D106 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 746: | Line 743: | ||
|align=center|3.5 | |align=center|3.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L122 |
|align=center| D107 | |align=center| D107 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 754: | Line 751: | ||
|align=center|6.5 | |align=center|6.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L123 |
|align=center| D107 | |align=center| D107 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 762: | Line 759: | ||
|align=center|6.5 | |align=center|6.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L124 |
|align=center| D102 | |align=center| D102 | ||
|align=center| D122 | |align=center| D122 | ||
| Line 770: | Line 767: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L125 |
|align=center| D102 | |align=center| D102 | ||
|align=center| D1118 | |align=center| D1118 | ||
| Line 778: | Line 775: | ||
|align=center|1 | |align=center|1 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L126 |
|align=center| D102 | |align=center| D102 | ||
|align=center| D114 | |align=center| D114 | ||
| Line 786: | Line 783: | ||
|align=center|7 | |align=center|7 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L127 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D119 | |align=center| D119 | ||
| Line 794: | Line 791: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C1 |
|align=center| D110 | |align=center| D110 | ||
|align=center| - | |align=center| - | ||
| Line 802: | Line 799: | ||
|align=center|14 | |align=center|14 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C2 |
|align=center| D129 | |align=center| D129 | ||
|align=center| - | |align=center| - | ||
| Line 810: | Line 807: | ||
|align=center|12 | |align=center|12 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C3 |
|align=center| D123 | |align=center| D123 | ||
|align=center| - | |align=center| - | ||
| Line 818: | Line 815: | ||
|align=center|12 | |align=center|12 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C4 |
|align=center| D103 | |align=center| D103 | ||
|align=center| - | |align=center| - | ||
| Line 826: | Line 823: | ||
|align=center|9 | |align=center|9 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C5 |
|align=center| D124 | |align=center| D124 | ||
|align=center| - | |align=center| - | ||
| Line 834: | Line 831: | ||
|align=center|10 | |align=center|10 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C6 |
|align=center| D104 | |align=center| D104 | ||
|align=center| - | |align=center| - | ||
| Line 842: | Line 839: | ||
|align=center|14 | |align=center|14 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C7 |
|align=center| D126 | |align=center| D126 | ||
|align=center| - | |align=center| - | ||
| Line 850: | Line 847: | ||
|align=center|14.5 | |align=center|14.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C8 |
|align=center| D105 | |align=center| D105 | ||
|align=center| - | |align=center| - | ||
| Line 858: | Line 855: | ||
|align=center|6 | |align=center|6 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C9 |
|align=center| D125 | |align=center| D125 | ||
|align=center| - | |align=center| - | ||
| Line 866: | Line 863: | ||
|align=center|15 | |align=center|15 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C10 |
|align=center| D106 | |align=center| D106 | ||
|align=center| - | |align=center| - | ||
| Line 874: | Line 871: | ||
|align=center|9.5 | |align=center|9.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C11 |
|align=center| D107 | |align=center| D107 | ||
|align=center| - | |align=center| - | ||
| Line 882: | Line 879: | ||
|align=center|13.5 | |align=center|13.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C12 |
|align=center| D102 | |align=center| D102 | ||
|align=center| - | |align=center| - | ||
| Line 891: | Line 888: | ||
|} | |} | ||
| + | == Purification of the B0034 and B0030 BioBricks == | ||
| - | + | ''We performed PCR on the B0034 BioBrick (MP 100) and the B0030 BioBrick (MP 120) to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.'' | |
| - | === Protocol === | + | === PCR Protocol === |
| - | For each samples, | + | ''For each samples,'' |
* 1 µl dNTP | * 1 µl dNTP | ||
| Line 904: | Line 902: | ||
* 1 µl Phusion | * 1 µl Phusion | ||
* 50 µl qsp H2O (33µl) | * 50 µl qsp H2O (33µl) | ||
| + | |||
| + | |||
| + | === Electrophoresis - Purification === | ||
| + | |||
| + | ''When the PCR cycles were finished,'' | ||
| + | |||
| + | * Add 10 µL of 6X loading dye. | ||
| + | * Load the samples (2 x 30 µL per sample) on a 1,5% agarose gel. | ||
| + | * Migration 30' at 100W. | ||
| + | |||
| + | |||
| + | ''After electrophoresis,'' | ||
| + | |||
| + | * Excision and purification of the bands corresponding to MP 100 and MP 120, using the QIAquick DNA Gel Extraction Kit (QIAGEN). | ||
| + | * Elution in 50 µL of water. | ||
| + | |||
| + | ''Because the intensity of the band corresponding to MP 120 was very low, we only continued with MP 100.'' | ||
| + | |||
| + | |||
| + | === DNA Digestion === | ||
| + | |||
| + | ''MP 100 was digested by EcoRI & SpeI (Forward Insert) or by XbaI & PstI (D100 : Backward Insert) | ||
| + | |||
| + | Digestion reaction (total volume : 50 µL)'' | ||
| + | |||
| + | * 25 µL DNA | ||
| + | * 5 µL buffer 2 (10X) | ||
| + | * 2 µL enzyme 1 | ||
| + | * 2 µL enzyme 2 | ||
| + | * 0.5 µL BSA (100X) | ||
| + | * 15,5 µL water | ||
| + | |||
| + | * The reaction was incubated 2 hours at 37°C. | ||
| + | * The samples (2 x 30 µL per sample) were then analysed by electrophoresis. | ||
| + | |||
| + | |||
| + | ===Electrophoresis=== | ||
| + | |||
| + | |||
| + | |||
| + | {| border="1" | ||
| + | |align=center|'''1''' | ||
| + | |align=center|'''2''' | ||
| + | |align=center|'''3''' | ||
| + | |align=center|'''4''' | ||
| + | |align=center|'''5''' | ||
| + | |align=center|'''6''' | ||
| + | |- | ||
| + | ! rowspan="1"|ladder | ||
| + | ! rowspan="1"|EcoRI & SpeI | ||
| + | ! rowspan="1"|EcoRI & SpeI | ||
| + | ! rowspan="1"| - | ||
| + | ! rowspan="1"|XbaI & PstI | ||
| + | ! rowspan="1"|XbaI & PstI | ||
| + | |} | ||
| + | |||
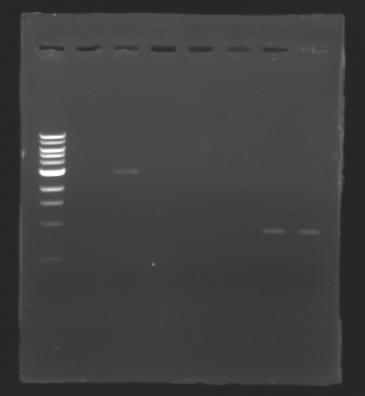
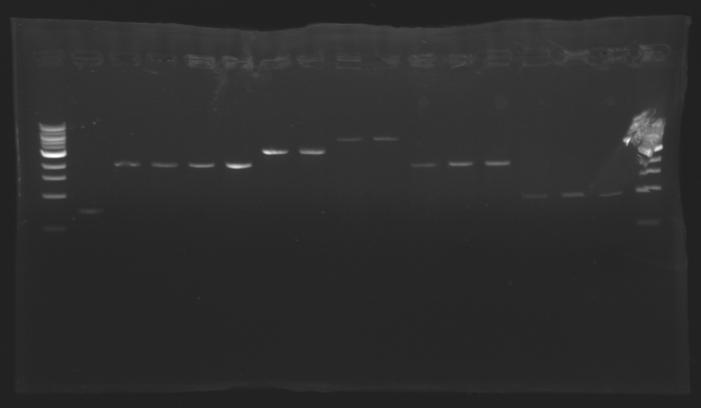
| + | [[Image:Image_gel.jpg|thumb|]] | ||
| + | |||
| + | The sequence of MP 100 (B0034) digested by EcoRI & SpeI (35 bp) or XbaI & PstI (34 bp) was too short and we didn't manage to visualise it on the gel. | ||
| + | |||
| + | ==> ''Conclusion'' : small parts like B0034 can't be cloned as an insert. | ||
Latest revision as of 16:59, 13 August 2008
|
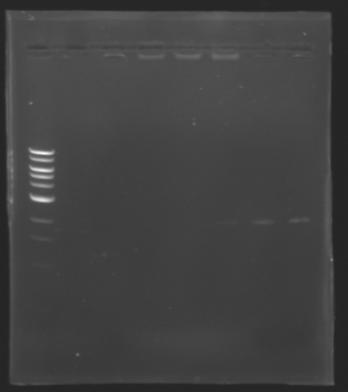
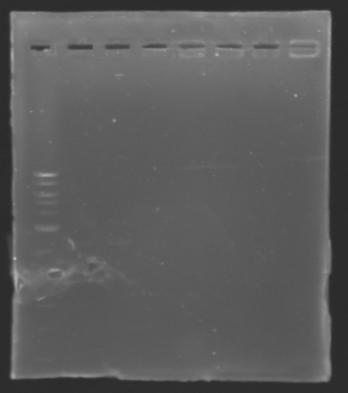
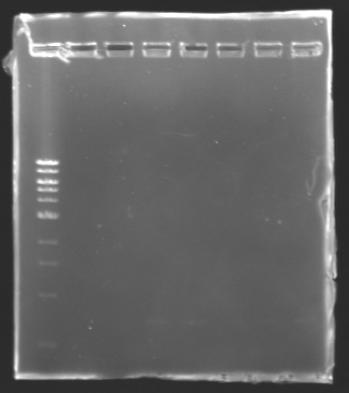
DNA ExtractionResults of the extraction : Electrophoresisconditions of electrophoresis:
LigationProtocolFor each samples,
List of ligations
Purification of the B0034 and B0030 BioBricksWe performed PCR on the B0034 BioBrick (MP 100) and the B0030 BioBrick (MP 120) to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments. PCR ProtocolFor each samples,
Electrophoresis - PurificationWhen the PCR cycles were finished,
Because the intensity of the band corresponding to MP 120 was very low, we only continued with MP 100.
DNA DigestionMP 100 was digested by EcoRI & SpeI (Forward Insert) or by XbaI & PstI (D100 : Backward Insert) Digestion reaction (total volume : 50 µL)
Electrophoresis
The sequence of MP 100 (B0034) digested by EcoRI & SpeI (35 bp) or XbaI & PstI (34 bp) was too short and we didn't manage to visualise it on the gel. ==> Conclusion : small parts like B0034 can't be cloned as an insert. |
 "
"