Team:Paris/July 30
From 2008.igem.org
(→Results of transformations) |
(→Results of transformations) |
||
| (9 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Paris/Calendar_Links|July 29|July 31}} | {{Paris/Calendar_Links|July 29|July 31}} | ||
| + | |||
| Line 9: | Line 10: | ||
|align="center"|'''Description''' | |align="center"|'''Description''' | ||
|align="center"|'''Antibio''' | |align="center"|'''Antibio''' | ||
| - | |align="center"|'''Nb colonies | + | |align="center"|'''Nb colonies''' |
|align="center"|'''Comments''' | |align="center"|'''Comments''' | ||
|- | |- | ||
|align="center"|L100 | |align="center"|L100 | ||
| - | |align="center"| | + | |align="center"|rbs TetR - ECFP<br>D110 (BV) - D130 (BI) |
| - | rbs TetR - ECFP | + | |
| - | <br>D110 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"|After another night nothing was osberved -> To do again | + | |align="center"|After another night nothing was osberved <br> -> To do again |
|- | |- | ||
|align="center"|L101 | |align="center"|L101 | ||
| - | |align="center"| | + | |align="center"|rbs TetR - GFP tripart<br>D110 (BV) - D131 (BI) |
| - | rbs TetR - GFP tripart | + | |
| - | <br>D110 (BV) - D131 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|1 | |align="center"|1 | ||
| - | |align="center"|After another night nothing was observed -> To do again | + | |align="center"|After another night nothing was observed <br> -> To do again |
|- | |- | ||
|align="center"|L102 | |align="center"|L102 | ||
| - | |align="center"| | + | |align="center"|Strong rbs - YFP<br>D129 (BV) - D118 (BI) |
| - | Strong rbs - YFP | + | |
| - | <br>D129 (BV) - D118 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L103 | |align="center"|L103 | ||
| - | |align="center"| | + | |align="center"|Strong rbs - mRFP<br>D129 (BV) - D122 (BI) |
| - | Strong rbs - | + | |
| - | <br>D129 (BV) - D122 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L104 | |align="center"|L104 | ||
| - | |align="center"| | + | |align="center"|Strong rbs - lasR activator<br>D129 (BV) - D114 (BI) |
| - | Strong rbs - lasR activator | + | |
| - | <br>D129 (BV) - D114 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L105 | |align="center"|L105 | ||
| - | |align="center"| | + | |align="center"|Strong promoter - ECFP<br>D123 (BV) - D130 (BI) |
| - | Strong promoter - ECFP | + | |
| - | <br>D123 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L106 | |align="center"|L106 | ||
| - | |align="center"| | + | |align="center"|Strong promoter - gfp Tripart<br>D123 (BV) - D131 (BI) |
| - | Strong promoter - gfp Tripart | + | |
| - | <br>D123 (BV) - D131 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L107 | |align="center"|L107 | ||
| - | |align="center"| | + | |align="center"|Strongest promoter - ECFP<br>D103 (BV) - D130 (BI) |
| - | Strongest promoter - ECFP | + | |
| - | <br>D103 (BV) - | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
| - | |align="center"|L108 n°2 (the right one) | + | |align="center"|L108 n°2 <br>(the right one) |
| - | |align="center"| | + | |align="center"|Strong promoter - gfp Tripart<br>D103 (BV) - D131 (BI) |
| - | Strong promoter - gfp Tripart | + | |
| - | <br>D103 (BV) - | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L109 n°1 | |align="center"|L109 n°1 | ||
| - | |align="center"| | + | |align="center"|Strong promoter - ecfp<br>D124 (BV) - D130 (BI) |
| - | Strong promoter - ecfp | + | |
| - | <br>D124 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 30 | |align="center"|+/- 30 | ||
| Line 93: | Line 74: | ||
|- | |- | ||
|align="center"|L109 n°2 | |align="center"|L109 n°2 | ||
| - | |align="center"| | + | |align="center"|Strong promoter - ecfp <br>D124 (BV) - D130 (BI) |
| - | Strong promoter - ecfp | + | |
| - | <br>D124 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| Line 101: | Line 80: | ||
|- | |- | ||
|align="center"|L110 | |align="center"|L110 | ||
| - | |align="center"| | + | |align="center"|Medium promoter - gfp Tripart<br>D124 (BV) - D131 (BI) |
| - | Medium promoter - gfp Tripart | + | |
| - | <br>D124 (BV) - D131 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L111 | |align="center"|L111 | ||
| - | |align="center"| | + | |align="center"|Weak promoter - ECFP<br>D104 (BV) - D130 (BI) |
| - | Weak promoter - ECFP | + | |
| - | <br>D104 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L112 | |align="center"|L112 | ||
| - | |align="center"| | + | |align="center"|Weak promoter - gfp tripart<br>D104 (BV) - D131 (BI) |
| - | Weak promoter - gfp | + | |
| - | <br>D104 (BV) - D131 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L113 | |align="center"|L113 | ||
| - | |align="center"| | + | |align="center"|AracpBAD - ecfp<br>D126 (BV) - D130 (BI) |
| - | AracpBAD - ecfp | + | |
| - | <br>D126 (BV) - D130 (BI) | + | |
|align="center"|Kan | |align="center"|Kan | ||
|align="center"|1 | |align="center"|1 | ||
| - | |align="center"|After another night nothing was observed -> To do again | + | |align="center"|After another night nothing was observed <br> -> To do again |
|- | |- | ||
|align="center"|L114 | |align="center"|L114 | ||
| - | |align="center"| | + | |align="center"|AracpBAD - gfp tripart<br>D126 (BV) - D131 (BI) |
| - | AracpBAD - gfp tripart | + | |
| - | <br>D126 (BV) - D131 (BI) | + | |
|align="center"|Kan | |align="center"|Kan | ||
|align="center"|2 | |align="center"|2 | ||
| - | |align="center"|After another night nothing was observed -> To do again | + | |align="center"|After another night nothing was observed <br>-> To do again |
|- | |- | ||
|align="center"|L115 | |align="center"|L115 | ||
| - | |align="center"| | + | |align="center"|pLas - ECFP<br>D105 (BV) - D130 (BI) |
| - | pLas - ECFP | + | |
| - | <br>D105 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L116 | |align="center"|L116 | ||
| - | |align="center"| | + | |align="center"|pLas - gfp Tripart<br>D105 (BV) - D131 (BI) |
| - | pLas - gfp Tripart | + | |
| - | <br>D105 (BV) - D131 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L117 | |align="center"|L117 | ||
| - | |align="center"| | + | |align="center"|yfp - Double Terminator<br>D117 (FI) - D125 (FV) |
| - | yfp - Double Terminator | + | |
| - | <br>D117 (FI) - D125 (FV) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 30 | |align="center"|+/- 30 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L118 | |align="center"|L118 | ||
| - | |align="center"| | + | |align="center"|rfp - Double Terminator<br>D121 (FI) - D125 (FV) |
| - | rfp - Double Terminator | + | |
| - | <br>D121 (FI) - D125 (FV) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 30 | |align="center"|+/- 30 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L119 | |align="center"|L119 | ||
| - | |align="center"| | + | |align="center"|lasR activator + LVA - Double Terminator<br>D113 (FI) - D125 (FV) |
| - | lasR activator + LVA - Double Terminator | + | |
| - | <br>D113 (FI) - D125 (FV) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 30 | |align="center"|+/- 30 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L120 | |align="center"|L120 | ||
| - | |align="center"| | + | |align="center"|tetR repressible promoter - ECFP<br>D106 (BV) - D130 (BI) |
| - | tetR repressible - ECFP | + | |
| - | <br>D106 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L121 | |align="center"|L121 | ||
| - | |align="center"| | + | |align="center"|tetR repressible promoter - gfp tripart<br>D106 (BV) - D131 (BI) |
| - | + | ||
| - | <br>D106 (BV) - | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L122 | |align="center"|L122 | ||
| - | |align="center"| | + | |align="center"|RBS-lasI - ecfp<br>D107 (BV) - D130 (BI) |
| - | RBS-lasI - ecfp | + | |
| - | <br>D107 (BV) - D130 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"|After another night nothing was observed -> To do again | + | |align="center"|After another night nothing was observed <br>-> To do again |
|- | |- | ||
|align="center"|L123 | |align="center"|L123 | ||
| - | |align="center"| | + | |align="center"|RBS lasI - gfp tripart<br>D107 (BV) - D131 (BI) |
| - | RBS lasI - | + | |
| - | <br>D107 (BV) - D131 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|1 | |align="center"|1 | ||
| - | |align="center"|After another night nothing was observed -> To do again | + | |align="center"|After another night nothing was observed <br>-> To do again |
|- | |- | ||
|align="center"|L124 | |align="center"|L124 | ||
| - | |align="center"| | + | |align="center"|Strongest RBS - mRFP<br>D102 (BV) - D122 (BI) |
| - | Strongest RBS - | + | |
| - | <br>D102 (BV) - D122 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L125 | |align="center"|L125 | ||
| - | |align="center"| | + | |align="center"|Strongest RBS - yfp<br>D102 (BV) - D118 (BI) |
| - | Strongest RBS - yfp | + | |
| - | <br>D102 (BV) - D118 (BI) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L126 | |align="center"|L126 | ||
| - | |align="center"| | + | |align="center"|Strongest RBS (1)- LacR activator (+LVA)<br>D102 (BV) - D114 (BI) |
| - | Strongest RBS (1)- LacR activator (+LVA) | + | |
| - | <br>D102 (BV) - | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|no dish found!!! | |align="center"|no dish found!!! | ||
| Line 237: | Line 182: | ||
|- | |- | ||
|align="center"|L127 | |align="center"|L127 | ||
| - | |align="center"| | + | |align="center"|gfp (1)- Double terminator<br>D119 (FI) - D125 (FV) |
| - | gfp (1)- Double terminator | + | |
| - | <br>D119 (FI) - D125 (FV) | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|no dish found!!! | |align="center"|no dish found!!! | ||
| Line 251: | Line 194: | ||
|- | |- | ||
|align="center"|C1 | |align="center"|C1 | ||
| - | |align="center"| | + | |align="center"|D110 |
| - | D110 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C2 | |align="center"|C2 | ||
| - | |align="center"| | + | |align="center"|D129 |
| - | D129 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|5 | |align="center"|5 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C3 | |align="center"|C3 | ||
| - | |align="center"| | + | |align="center"|D123 |
| - | D123 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C4 | |align="center"|C4 | ||
| - | |align="center"| | + | |align="center"|D103 |
| - | D103 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|+/- 20 | |align="center"|+/- 20 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C5 | |align="center"|C5 | ||
| - | |align="center"| | + | |align="center"|D124 |
| - | D124 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C6 | |align="center"|C6 | ||
| - | |align="center"| | + | |align="center"|D104 |
| - | D104 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C7 | |align="center"|C7 | ||
| - | |align="center"| | + | |align="center"|D126 |
| - | D126 | + | |align="center"|Kana |
| - | |align="center"| | + | |
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C8 | |align="center"|C8 | ||
| - | |align="center"| | + | |align="center"|D105 |
| - | D105 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|+/- 20 | |align="center"|+/- 20 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C9 | |align="center"|C9 | ||
| - | |align="center"| | + | |align="center"|D125 |
| - | D125 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C10 | |align="center"|C10 | ||
| - | |align="center"| | + | |align="center"|D106 |
| - | D106 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C11 | |align="center"|C11 | ||
| - | |align="center"| | + | |align="center"|D107 |
| - | D107 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C12 | |align="center"|C12 | ||
| - | |align="center"| | + | |align="center"|D102 |
| - | D102 | + | |align="center"|Amp |
| - | |align="center"| | + | |
|align="center"|+/- 10 | |align="center"|+/- 10 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|Positive control | |align="center"|Positive control | ||
| - | |align="center"| | + | |align="center"|puc19 |
| - | puc19 | + | |align="center"|Amp |
| - | |align="center"| | + | |align="center"|155 (transformation <br> efficiency:1.5*10^7/ug) |
| - | |align="center"|155 (transformation efficiency:1.5*10^7/ug) | + | |align="center"|ok |
| - | |align="center"| | + | |
|} | |} | ||
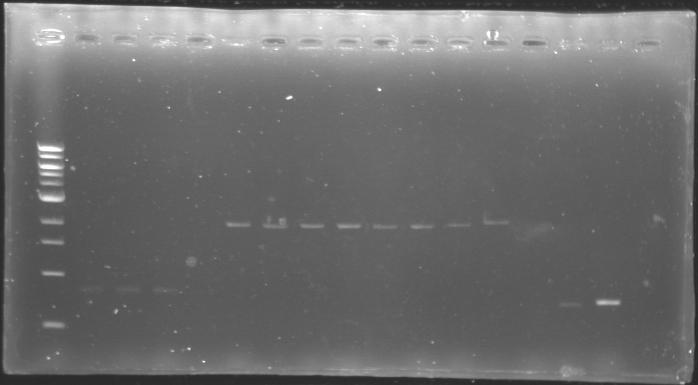
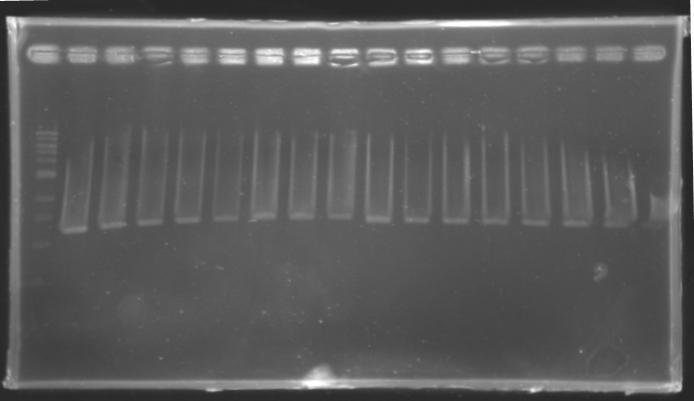
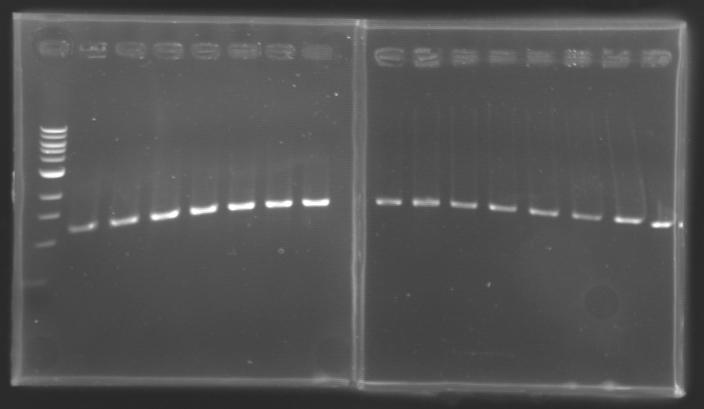
==Analysis of yesterday DNA digestion== | ==Analysis of yesterday DNA digestion== | ||
| - | The digested DNA of yesterday was analysed one more time by electrophoresis on a 0.8% agarose gel (about 30 minutes at 100 W). The ladder used was the 1 kb DNA ladder (New England Biolabs). 5 µL of each sample with 1 µL of loading dye were loaded. | + | ''The digested DNA of yesterday was analysed '''one more time''' by electrophoresis on a '''0.8% agarose gel''' (about 30 minutes at 100 W).'' |
| + | * The ladder used was the 1 kb DNA ladder (New England Biolabs). | ||
| + | * 5 µL of each sample with 1 µL of loading dye were loaded.' | ||
[[Image:KR000082.jpg|thumb|]] | [[Image:KR000082.jpg|thumb|]] | ||
| - | |||
| - | |||
{| border="1" | {| border="1" | ||
| Line 485: | Line 415: | ||
|} | |} | ||
| - | + | ==> '''Conclusion :'''Each of the samples was succesfully digested and purified except for the sample D108. It seems that the QIAprep columms (from the QIAGEN Minipreps kit) can be used instead of the QIAquick columms (for DNA Gel Extraction). | |
| - | Each of the samples was succesfully digested and purified except for the sample D108. It seems that the QIAprep columms (from the QIAGEN Minipreps kit) can be used instead of the QIAquick columms (for DNA Gel Extraction). | + | |
| + | |||
| + | |||
| + | == '''PCR Screening of Ligation Transformants'''== | ||
| + | |||
| + | Use of 8 clones of Ligation transformants for screening PCR | ||
| + | |||
| + | |||
| + | ===Protocol of screening PCR=== | ||
| + | |||
| + | * '''Mix''' | ||
| + | {| Border="1" | ||
| + | |align="center"|'''Name''' | ||
| + | |align="center"|'''Vol (µl)''' | ||
| + | |align="center"|'''Concentration''' | ||
| + | |- | ||
| + | |align="center"|Quick Load | ||
| + | |align="center"|25µl | ||
| + | |align="center"|2X | ||
| + | |- | ||
| + | |align="center"|OligoF_VF2 (O18) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|OligoR_VR (O19) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|water | ||
| + | |align="center"|23µl | ||
| + | |- | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | * 50µl of Mix PCR by tube/clone | ||
| + | * one toothpick of each clone's colony by tube | ||
| + | * Program : Annealing 55°C - Time élongation 1'30" - Number cycle : 29 | ||
| + | |||
| + | |||
| + | === Conditions of electrophoresis === | ||
| + | |||
| + | |||
| + | * 10µl of ladder 1 kb | ||
| + | * 15µl of screening PCR (gel n°1, 2, 3(9-17), 4, 5, 6, 7, 8, 9, 10, 11) | ||
| + | * 10µl of screening PCR (gel n°3(1), 13, 14) | ||
| + | * migration ~30min at 100W on '''0,8%''' gel | ||
| + | |||
| + | |||
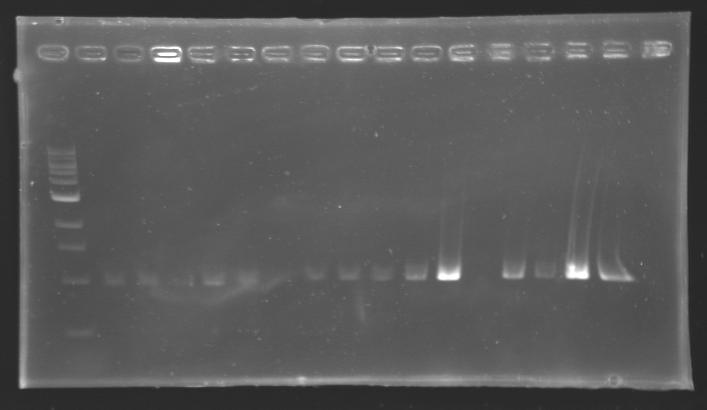
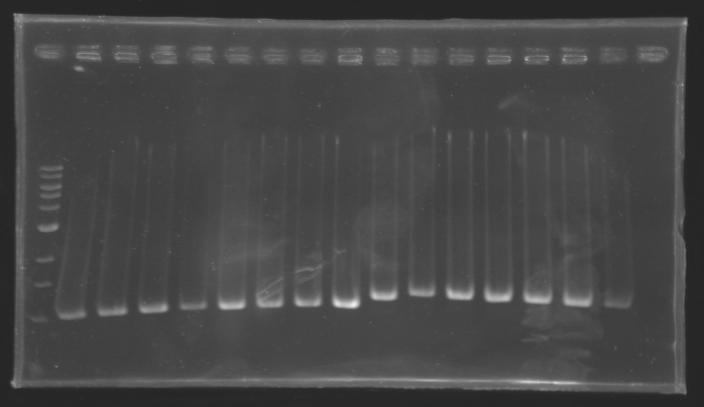
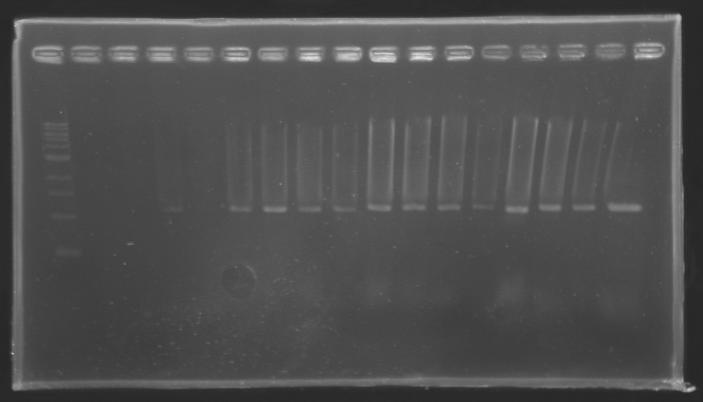
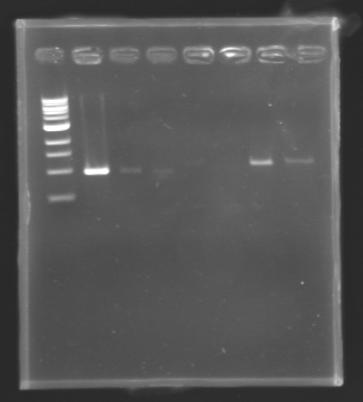
| + | ===Results=== | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
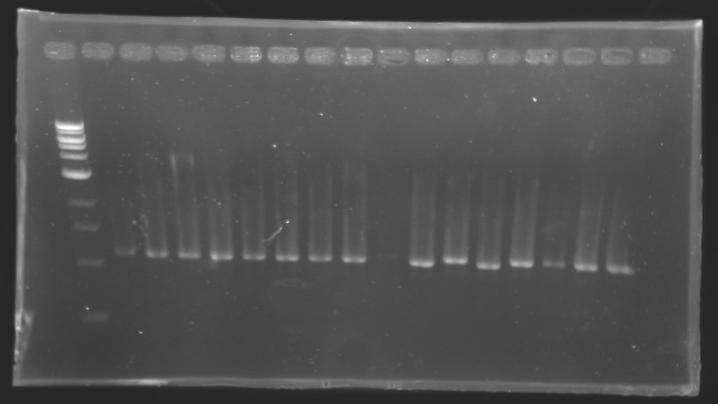
| + | |colspan="3"|PCR1_’’’L102(1-8)’’’ | ||
| + | |colspan="3"|PCR2_’’’L103(1-8)’’’ | ||
| + | |colspan="3"|PCR3_’’’L104(1-8)’’’ | ||
| + | |colspan="3"|PCR4_’’’L105(1-8)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1045 pb | ||
| + | |align="center"|1000 pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"|1003 pb | ||
| + | |align="center"|1000 pb | ||
| + | |align="center"|10-->17 | ||
| + | |align="center"| 1078 pb | ||
| + | |align="center"| 1000pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"|10-->17 | ||
| + | |- | ||
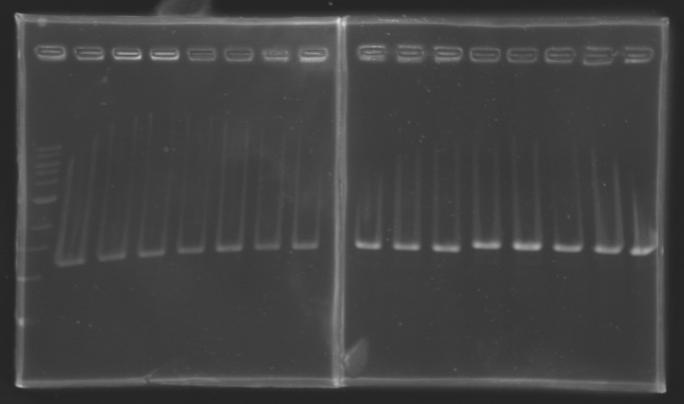
| + | |colspan="6"|[[Image: KR000084_1.jpg|thumb|'''Gel 1 : L102-L103''']] | ||
| + | |colspan="6"|[[Image: KR000085_2.jpg|thumb|'''Gel 2 : L104-L105''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
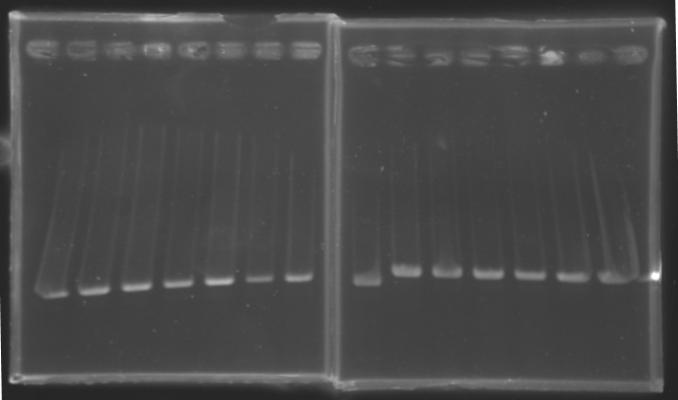
| + | {| border="1" | ||
| + | |colspan="3"|PCR5_’’’L106(1-8)’’’ | ||
| + | |colspan="3"|PCR6_’’’L107(1-8)’’’ | ||
| + | |colspan="3"|PCR7_’’’L108.1(1-8)’’’ | ||
| + | |colspan="3"|PCR8_’’’L108.2(1-8)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"|10-->17 | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"|10-->17 | ||
| + | |- | ||
| + | |colspan="6"|[[Image: KR000087_3.jpg|thumb|'''Gel 3 : L106-L107''']] | ||
| + | |colspan="6"|[[Image: KR000089_4.jpg|thumb|'''Gel 4 : L108.1-L108.2''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
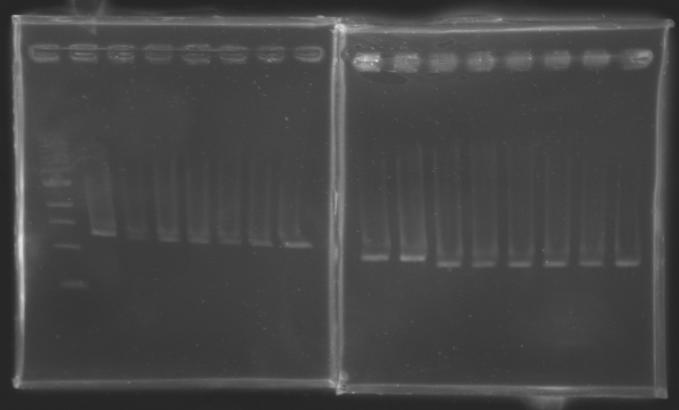
| + | {| border="1" | ||
| + | |colspan="3"|PCR9_’’’L110(1-8)’’’ | ||
| + | |colspan="3"|PCR10_’’’L111(1-8)’’’ | ||
| + | |colspan="3"|PCR11_’’’L112(1-8)’’’ | ||
| + | |colspan="3"|PCR12_’’’L115(1-7)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"| 1100 pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"| 1100 pb | ||
| + | |align="center"|10-->17 | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|2-->10 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"|700 pb | ||
| + | |align="center"|11-->16 | ||
| + | |- | ||
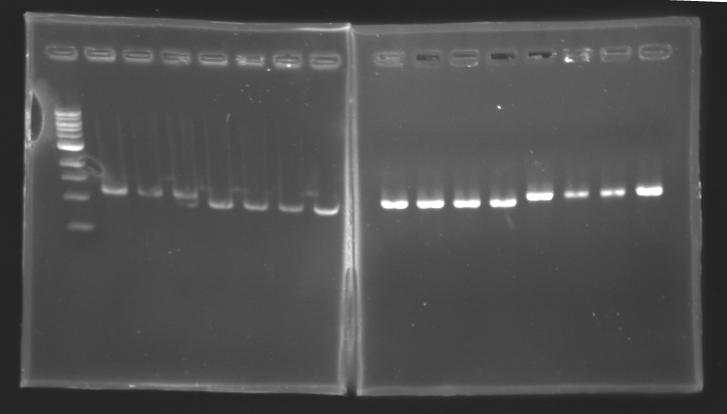
| + | |colspan="6"|[[Image: KR000090_5.jpg|thumb|'''Gel 5 : L110-L111''']] | ||
| + | |colspan="6"|[[Image: KR000091_6.jpg|thumb|'''Gel 6 : L112-L115''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR13_’’’L115(8)’’’ | ||
| + | |colspan="3"|PCR14_’’’L116(1-8)’’’ | ||
| + | |colspan="3"|PCR15_’’’L117(1-6)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|2 | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|3-->10 | ||
| + | |align="center"|1046 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|11-->16 | ||
| + | |- | ||
| + | |colspan="6"|[[Image: KR000092_7.jpg|thumb|'''Gel 7 : L115-L116-L117''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR16_’’’L117(7-8)’’’ | ||
| + | |colspan="3"|PCR17_’’’L118(1-8)’’’ | ||
| + | |colspan="3"|PCR18_’’’L119(1-5)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1046 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|2-->3 | ||
| + | |align="center"|1004 pb | ||
| + | |align="center"| 1100 pb | ||
| + | |align="center"|4-->11 | ||
| + | |align="center"|1079 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|12-->16 | ||
| + | |- | ||
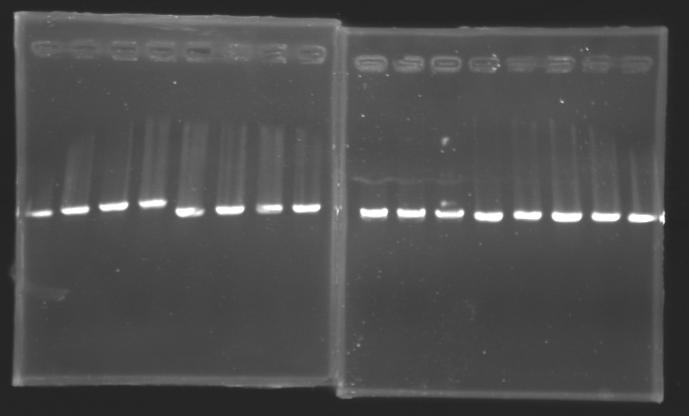
| + | |colspan="6"|[[Image: KR000093_8.jpg|thumb|'''Gel 8 : L117-L118-L119''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR19_’’’L119(6-8)’’’ | ||
| + | |colspan="3"|PCR20_’’’L120(1-8)’’’ | ||
| + | |colspan="3"|PCR21_’’’L121(1-4)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1079 pb | ||
| + | |align="center"| 1100 pb | ||
| + | |align="center"|2-->4 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|5-->12 | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|13-->16 | ||
| + | |- | ||
| + | |colspan="6"|[[Image: KR000094_9.jpg|thumb|'''Gel 9 : L119-L120-L121''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR22_’’’L121(5-8)’’’ | ||
| + | |colspan="3"|PCR23_’’’L122(1-8)’’’ | ||
| + | |colspan="3"|PCR24_’’’L125(1-4)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|2-->5 | ||
| + | |align="center"|1239 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|6-->12 | ||
| + | |align="center"|1045 pb | ||
| + | |align="center"| 1000pb | ||
| + | |align="center"|13-->16 | ||
| + | |- | ||
| + | |colspan="6"|[[Image: KR000095_10.jpg|thumb|'''Gel 10 : L121-L122-L125''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR25_’’’L125(5-8)’’’ | ||
| + | |colspan="3"|PCR26_’’’L109.1(1-7)’’’ | ||
| + | |colspan="3"|PCR27_’’’L109.2(1-8)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1045 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|2-->5 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"| 1100 pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"| 1100 pb | ||
| + | |align="center"|10-->17 | ||
| + | |- | ||
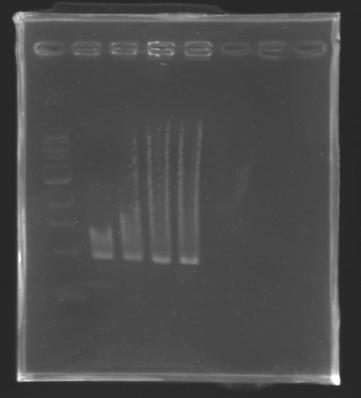
| + | |colspan="3"|[[Image: KR000096_11.jpg|thumb|'''Gel 11 : L125''']] | ||
| + | |colspan="6"|[[Image: KR000099_14.jpg|thumb|'''Gel 14 : L109.1-L109.2''']] | ||
| + | |} | ||
| + | |||
| + | |||
| + | ==> '''Conclusion :''' with the PCR, we have check that the transformant bacteria contain insert. (obtain amplification at the good size). | ||
| + | |||
| + | But we don't observe results for L102(3), L102(6), L103(4), L106(1), L106(2), L106(4), L111(1) | ||
| + | |||
| + | Migration of an another gel for this sample... | ||
| + | |||
| + | |||
| + | '''Results''': | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR1_’’’L102(3;6))’’’ | ||
| + | |colspan="3"|PCR2_’’’L103(4)’’’ | ||
| + | |colspan="3"|PCR5_’’’L106(1; 2; 4)’’’ | ||
| + | |colspan="3"|PCR10_’’’L111(1)’’’ | ||
| + | |||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
| + | |align="center"| 1045 pb | ||
| + | |align="center"| 1000 pb | ||
| + | |align="center"|2-3 | ||
| + | |align="center"|1003 pb | ||
| + | |align="center"|- | ||
| + | |align="center"|4 | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"|1100 pb | ||
| + | |align="center"|5-6-7 | ||
| + | |align="center"|1239 pb | ||
| + | |align="center"|1100 pb | ||
| + | |align="center"|8 | ||
| + | |- | ||
| + | |colspan="6"|[[Image: KR000098_13.jpg|thumb|'''Gel 13 : to solve Mistakes''']] | ||
| + | |} | ||
| + | |||
| + | |||
| + | ==> '''Conclusion :''' We can observe a results for the samples : L102, L103, L106(4) and L111. | ||
| + | (but not for L106(1; 2)) | ||
Latest revision as of 17:03, 13 August 2008
|
Results of transformations
Analysis of yesterday DNA digestionThe digested DNA of yesterday was analysed one more time by electrophoresis on a 0.8% agarose gel (about 30 minutes at 100 W).
==> Conclusion :Each of the samples was succesfully digested and purified except for the sample D108. It seems that the QIAprep columms (from the QIAGEN Minipreps kit) can be used instead of the QIAquick columms (for DNA Gel Extraction).
PCR Screening of Ligation TransformantsUse of 8 clones of Ligation transformants for screening PCR
Protocol of screening PCR
Conditions of electrophoresis
Results
But we don't observe results for L102(3), L102(6), L103(4), L106(1), L106(2), L106(4), L111(1) Migration of an another gel for this sample...
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"