Team:Paris/August 11
From 2008.igem.org
(→PCR amplification) |
(→Culture of ligation transformants) |
||
| (17 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
==Transformation== | ==Transformation== | ||
| + | All the ligations were transformed according [[Team:Paris/Notebook/Protocols#Transformation|transformation for Top10 protocol]] | ||
| + | |||
| - | |||
| - | |||
==PCR== | ==PCR== | ||
| Line 116: | Line 116: | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
|PCR_130 | |PCR_130 | ||
| - | | | + | |E0240 RBS+ |
|O141_O140 | |O141_O140 | ||
| - | | | + | |MP143 |
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_130' |
| - | | - | ||
|O141_O140 | |O141_O140 | ||
|Water | |Water | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_131 |
|flhD RBS- | |flhD RBS- | ||
|O132_O133 | |O132_O133 | ||
|MG1655 | |MG1655 | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_131' |
| - | | - | ||
|O132_O133 | |O132_O133 | ||
|Water | |Water | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_132 |
|flhC RBS- | |flhC RBS- | ||
|O130_O131 | |O130_O131 | ||
|MG1655 | |MG1655 | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_132' |
| - | | - | ||
|O130_O131 | |O130_O131 | ||
|Water | |Water | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_133 |
| - | | | + | |flhDC + prom |
|O110_O131 | |O110_O131 | ||
|MG1655 | |MG1655 | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_133' |
| - | | - | ||
|O110_O131 | |O110_O131 | ||
|Water | |Water | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_134 |
| - | | | + | |flhDC + prom |
|O111_O131 | |O111_O131 | ||
|MG1655 | |MG1655 | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_134' |
| - | | - | ||
|O111_O131 | |O111_O131 | ||
|Water | |Water | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_135 |
|pfliL | |pfliL | ||
|O124_O125 | |O124_O125 | ||
|MG1655 | |MG1655 | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_135' |
| - | | - | ||
|O124_O125 | |O124_O125 | ||
|Water | |Water | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_136 |
|pflhDC | |pflhDC | ||
|O110_O113 | |O110_O113 | ||
|MG1655 | |MG1655 | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_136' |
| - | | - | ||
|O110_O113 | |O110_O113 | ||
|Water | |Water | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_137 |
|pflhDC | |pflhDC | ||
|O111_O113 | |O111_O113 | ||
|MG1655 | |MG1655 | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_137' |
| - | | - | ||
|O111_O113 | |O111_O113 | ||
| Line 198: | Line 198: | ||
* Program PCR_Screening : Annealing 30" at 60°C - Time élongation 1'30" at 72°C - Number cycle : 30 | * Program PCR_Screening : Annealing 30" at 60°C - Time élongation 1'30" at 72°C - Number cycle : 30 | ||
| - | |||
==== '''PCR verification/Analysis''' ==== | ==== '''PCR verification/Analysis''' ==== | ||
| Line 225: | Line 224: | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
|PCR_130 | |PCR_130 | ||
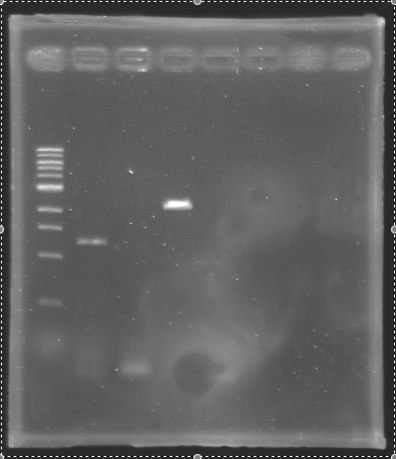
| - | | | + | |E0240 |
|Gel 1 | |Gel 1 | ||
|2 | |2 | ||
| Line 231: | Line 230: | ||
|style="background: #cbff7B"|<center> 900 bp </center> | |style="background: #cbff7B"|<center> 900 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_130' |
|Negative Control | |Negative Control | ||
|Gel 1 | |Gel 1 | ||
| Line 238: | Line 237: | ||
|style="background: #cbff7B"|<center> 0 bp </center> | |style="background: #cbff7B"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_131 |
|flhD RBS- | |flhD RBS- | ||
|Gel 1 | |Gel 1 | ||
| Line 245: | Line 244: | ||
|style="background: #cbff7B"|<center> 350 bp </center> | |style="background: #cbff7B"|<center> 350 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_131' |
|Negative Control | |Negative Control | ||
|Gel 1 | |Gel 1 | ||
| Line 252: | Line 251: | ||
|style="background: #cbff7B"|<center> 0 bp </center> | |style="background: #cbff7B"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_132 |
|flhC RBS- | |flhC RBS- | ||
|Gel 1 | |Gel 1 | ||
| Line 259: | Line 258: | ||
|style="background: #cbff7B"|<center> 600 bp </center> | |style="background: #cbff7B"|<center> 600 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_132' |
|Negative Control | |Negative Control | ||
|Gel 1 | |Gel 1 | ||
| Line 266: | Line 265: | ||
|style="background: #cbff7B"|<center> 0 bp </center> | |style="background: #cbff7B"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_133 |
| - | | | + | |flhDC + prom |
|Gel 2 | |Gel 2 | ||
|2 | |2 | ||
| Line 273: | Line 272: | ||
|style="background: #cbff7B"|<center> 1300 bp </center> | |style="background: #cbff7B"|<center> 1300 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_133' |
|Negative Control | |Negative Control | ||
|Gel 2 | |Gel 2 | ||
| Line 280: | Line 279: | ||
|style="background: #cbff7B"|<center> 0 bp </center> | |style="background: #cbff7B"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_134 |
| - | | | + | |flhDC + prom |
|Gel 2 | |Gel 2 | ||
|4 | |4 | ||
| Line 287: | Line 286: | ||
|style="background: #ff6d73"|<center> 2100 pb </center> | |style="background: #ff6d73"|<center> 2100 pb </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_134' |
|Negative Control | |Negative Control | ||
|Gel 2 | |Gel 2 | ||
| Line 296: | Line 295: | ||
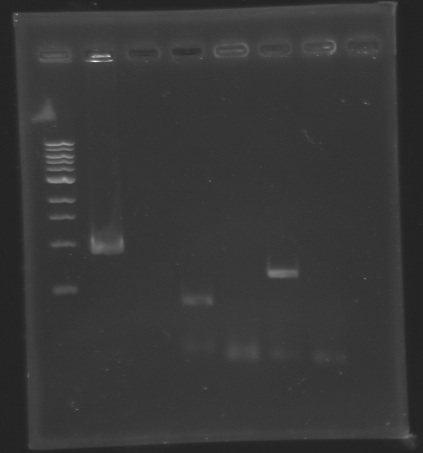
| - | ==> '''Conclusion :''' We observed the size expected for the PCR products, but not for pflhDC ( | + | ==> '''Conclusion :''' We observed the size expected for the PCR products, but not for pflhDC (PCR_134), is right. We hypothesis for PCR_138 that the size is longer that expected due to the aspecific fixation of Oligo O111 (upper to the real site). |
* '''Electrophoresis''' | * '''Electrophoresis''' | ||
| - | + | [[Image:KR000150.jpg|thumb|Analysis of PCR product (Gel 3)]] | |
ladder : 10µl ladder 100 bp | ladder : 10µl ladder 100 bp | ||
<br> samples : 3µl of PCR products + 2µl of Loading Dye | <br> samples : 3µl of PCR products + 2µl of Loading Dye | ||
| Line 316: | Line 315: | ||
|align="center"|'''Measured size''' | |align="center"|'''Measured size''' | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_135 |
|pfliL | |pfliL | ||
|Gel 3 | |Gel 3 | ||
|2 | |2 | ||
| - | | | + | |124 bp |
|style="background: #ff6d73"|<center> 0 bp </center> | |style="background: #ff6d73"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_135' |
|Negative Control | |Negative Control | ||
|Gel 3 | |Gel 3 | ||
| Line 330: | Line 329: | ||
|style="background: #cbff7B"|<center> 0 bp </center> | |style="background: #cbff7B"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_136 |
|pflhDC | |pflhDC | ||
|Gel 3 | |Gel 3 | ||
|4 | |4 | ||
| - | | | + | |223 |
|style="background: #ff6d73"|<center> 0 bp </center> | |style="background: #ff6d73"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_136' |
|Negative Control | |Negative Control | ||
|Gel 3 | |Gel 3 | ||
| Line 344: | Line 343: | ||
|style="background: #cbff7B"|<center> 0 bp </center> | |style="background: #cbff7B"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_137 |
|pflhDC | |pflhDC | ||
|Gel 3 | |Gel 3 | ||
|6 | |6 | ||
| - | | | + | |369 |
|style="background: #ff6d73"|<center> 0 bp </center> | |style="background: #ff6d73"|<center> 0 bp </center> | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |PCR_137' |
|Negative Control | |Negative Control | ||
|Gel 3 | |Gel 3 | ||
| Line 359: | Line 358: | ||
|} | |} | ||
| - | ==Culture of ligation transformants== | + | |
| + | ==> '''Conclusion :''' We need to repeat the experiments. | ||
| + | |||
| + | ==Culture of ligation transformants (pFlgA, pFlgB and pFlhB)== | ||
*4 clones of each transformation were cultured in '''7,5 mL LB + ampicilline'''. The colonies picked up were the rest of those already picked up from the transformation plate (for the PCR screening of August 8). | *4 clones of each transformation were cultured in '''7,5 mL LB + ampicilline'''. The colonies picked up were the rest of those already picked up from the transformation plate (for the PCR screening of August 8). | ||
Latest revision as of 16:11, 18 August 2008
TransformationAll the ligations were transformed according transformation for Top10 protocol
PCRWe performed PCR on to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.
PCR amplificationProtocol
For each sample, 1 µl dNTP
PCR verification/AnalysisAfter the PCR :
ladder : 10µl ladder 1 kb
ladder : 10µl ladder 100 bp
Culture of ligation transformants (pFlgA, pFlgB and pFlhB)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"