TUDelft/4 September 2008
From 2008.igem.org
m (→3A Assembly) |
m (→September 4th 2008) |
||
| (2 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
{{Template:TUDelftiGEM2008_calendar}} | {{Template:TUDelftiGEM2008_calendar}} | ||
| - | =September 4th | + | =September 4th= |
==Transformation== | ==Transformation== | ||
| Line 10: | Line 10: | ||
==PCR== | ==PCR== | ||
| - | A new PCR with the <i>Pfx</i> enzyme was performed on the <i>E.coli</i> genes. This time more template DNA was used as the last times this PCR was performed it yielded no results. Two reactions of 50 ul were set up as last time. 50ng of template (genomic <i>E. coli</i> DNA) was used, other characteristics were the same. | + | A new PCR with the <i>Pfx</i> enzyme was performed on the <i>E.coli</i> genes. This time more template DNA was used as the [https://2008.igem.org/TUDelft/21_August_2008 last times] this PCR was performed it yielded no results. Two reactions of 50 ul were set up as last time. 50ng of template (genomic <i>E. coli</i> DNA) was used, other characteristics were the same. |
[[Image:TUDelft040908PCR.jpg|thumb|center|Picture of the gel on which PCR products were run. A band was cut out (circled area).]] | [[Image:TUDelft040908PCR.jpg|thumb|center|Picture of the gel on which PCR products were run. A band was cut out (circled area).]] | ||
| - | Strangely, only one of the reactions yielded a result, for the | + | Strangely, only one of the reactions yielded a result, for the idi gene. The picture of the gel with band cut out can be seen above. At this point, it is not clear what went wrong. |
==Double restriction== | ==Double restriction== | ||
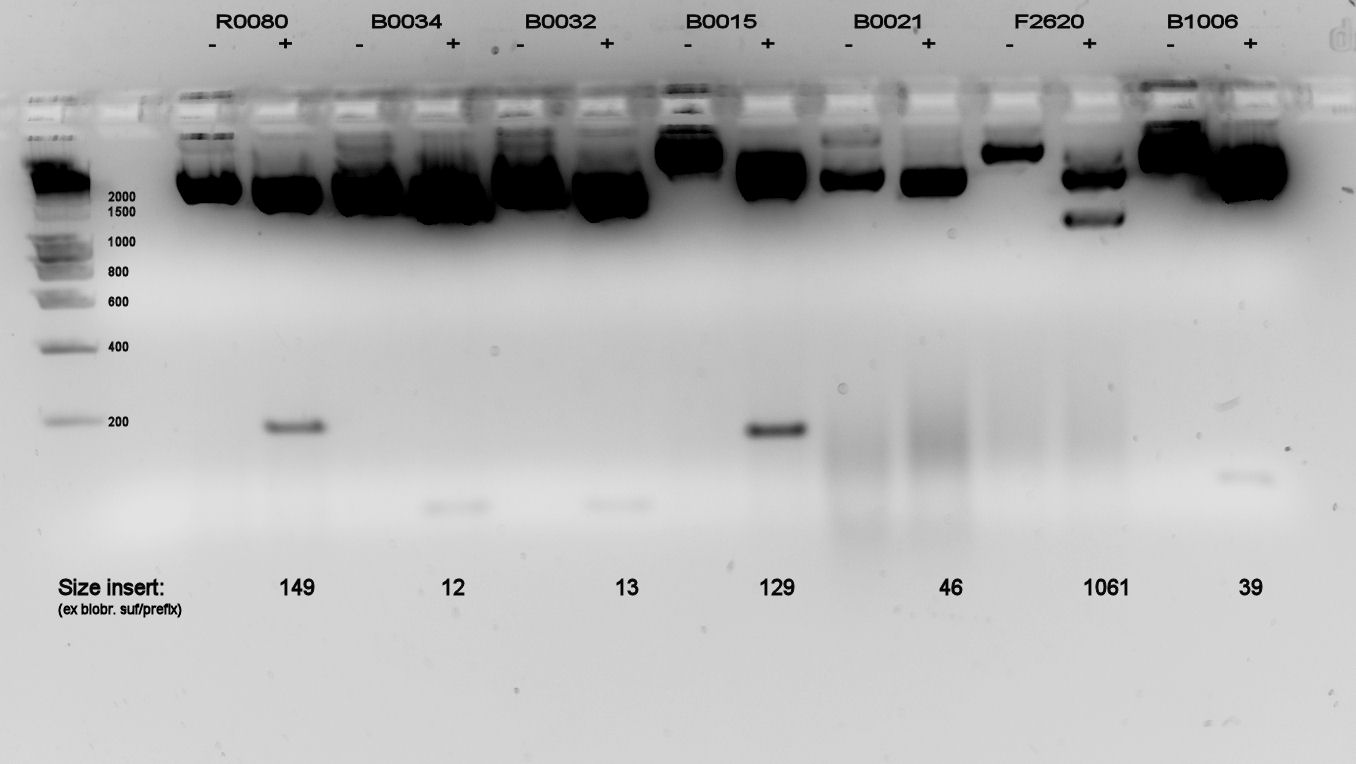
| - | We did a double restriction ( | + | We did a double restriction (''Eco''RI & ''Pst''I) on the newly received living stabs, and ran a 2% agarose gel, because there were many parts < 200 bp. The gel is displayed in figure 1. |
| - | [[Image:TUDelft040908doublerestriction.jpg|thumb|left|Figure 1: Midiprepped plasmids/parts from second set of living stabs. Digested by | + | [[Image:TUDelft040908doublerestriction.jpg|thumb|left|Figure 1: Midiprepped plasmids/parts from second set of living stabs. Digested by ''Eco''RI and ''Pst''I]] |
| - | Most parts look all right. Due to the small parts having low light emittance, we used a lot of DNA, causing overexposure for the backbones. Only parts B0021 and F2620, a terminator and promoter, showed a smear in both cut and uncut DNA. B0021 was indeterminable, F2620 was correct size. All the other parts were also likely to be correct size. | + | Most parts look all right. Due to the small parts having low mass and thus low light emittance, we used a lot of DNA, causing overexposure for the backbones. Only parts B0021 and F2620, a terminator and promoter, showed a smear in both cut and uncut DNA. B0021 was indeterminable, F2620 was correct size (which was also visible on the gel of the 2nd of September which was not posted). All the other parts were also likely to be correct size. |
{{Template:TUDelftiGEM2008_sidebar}} | {{Template:TUDelftiGEM2008_sidebar}} | ||
Latest revision as of 10:21, 27 October 2008
| July | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 | 31 | |||
| August | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | ||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 |
| September | ||||||
| M | T | W | T | F | S | S |
| [http://2008.igem.org/TUDelft/1_September_2008 1] | [http://2008.igem.org/TUDelft/2_September_2008 2] | [http://2008.igem.org/TUDelft/3_September_2008 3] | [http://2008.igem.org/TUDelft/4_September_2008 4] | [http://2008.igem.org/TUDelft/5_September_2008 5] | [http://2008.igem.org/wiki/index.php?title=TUDelft/6_September_2008&action=edit 6] | [http://2008.igem.org/wiki/index.php?title=TUDelft/7_September_2008&action=edit 7] |
| [http://2008.igem.org/TUDelft/8_September_2008 8] | [http://2008.igem.org/TUDelft/9_September_2008 9] | [http://2008.igem.org/TUDelft/10_September_2008 10] | [http://2008.igem.org/TUDelft/11_September_2008 11] | [http://2008.igem.org/TUDelft/12_September_2008 12] | [http://2008.igem.org/wiki/index.php?title=TUDelft/13_September_2008&action=edit 13] | [http://2008.igem.org/wiki/index.php?title=TUDelft/14_September_2008&action=edit 14] |
| [http://2008.igem.org/TUDelft/15_September_2008 15] | [http://2008.igem.org/TUDelft/16_September_2008 16] | [http://2008.igem.org/TUDelft/17_September_2008 17] | [http://2008.igem.org/TUDelft/18_September_2008 18] | [http://2008.igem.org/TUDelft/19_September_2008 19] | [http://2008.igem.org/wiki/index.php?title=TUDelft/20_September_2008&action=edit 20] | [http://2008.igem.org/wiki/index.php?title=TUDelft/21_September_2008&action=edit 21] |
| [http://2008.igem.org/TUDelft/22_September_2008 22] | [http://2008.igem.org/TUDelft/23_September_2008 23] | [http://2008.igem.org/TUDelft/24_September_2008 24] | [http://2008.igem.org/TUDelft/25_September_2008 25] | [http://2008.igem.org/wiki/index.php?title=TUDelft/26_September_2008&action=edit 26] | [http://2008.igem.org/wiki/index.php?title=TUDelft/27_September_2008&action=edit 27] | [http://2008.igem.org/wiki/index.php?title=TUDelft/28_September_2008&action=edit 28] |
| [http://2008.igem.org/TUDelft/29_September_2008 29] | [http://2008.igem.org/TUDelft/30_September_2008 30] | |||||
| October | ||||||
| M | T | W | T | F | S | S |
| [http://2008.igem.org/TUDelft/1_October_2008 1] | [http://2008.igem.org/TUDelft/2_October_2008 2] | [http://2008.igem.org/TUDelft/3_October_2008 3] | [http://2008.igem.org/wiki/index.php?title=TUDelft/4_October_2008&action=edit 4] | [http://2008.igem.org/wiki/index.php?title=TUDelft/5_October_2008&action=edit 5] | ||
| [http://2008.igem.org/TUDelft/6_October_2008 6] | [http://2008.igem.org/TUDelft/7_October_2008 7] | [http://2008.igem.org/TUDelft/8_October_2008 8] | [http://2008.igem.org/TUDelft/9_October_2008 9] | [http://2008.igem.org/TUDelft/10_October_2008 10] | [http://2008.igem.org/wiki/index.php?title=TUDelft/11_October_2008&action=edit 11] | [http://2008.igem.org/wiki/index.php?title=TUDelft/12_October_2008&action=edit 12] |
| [http://2008.igem.org/TUDelft/13_October_2008 13] | [http://2008.igem.org/TUDelft/14_October_2008 14] | [http://2008.igem.org/TUDelft/15_October_2008 15] | [http://2008.igem.org/TUDelft/16_October_2008 16] | [http://2008.igem.org/TUDelft/17_October_2008 17] | [http://2008.igem.org/wiki/index.php?title=TUDelft/18_October_2008&action=edit 18] | [http://2008.igem.org/wiki/index.php?title=TUDelft/19_October_2008&action=edit 19] |
| [http://2008.igem.org/TUDelft/20_October_2008 20] | [http://2008.igem.org/TUDelft/21_October_2008 21] | [http://2008.igem.org/TUDelft/22_October_2008 22] | [http://2008.igem.org/TUDelft/23_October_2008 23] | [http://2008.igem.org/TUDelft/24_October_2008 24] | [http://2008.igem.org/wiki/index.php?title=TUDelft/25_October_2008&action=edit 25] | [http://2008.igem.org/wiki/index.php?title=TUDelft/26_October_2008&action=edit 26] |
| [http://2008.igem.org/wiki/index.php?title=TUDelft/27_October_2008&action=edit 27] | [http://2008.igem.org/wiki/index.php?title=TUDelft/28_October_2008&action=edit 28] | [http://2008.igem.org/wiki/index.php?title=TUDelft/29_October_2008&action=edit 29] | [http://2008.igem.org/wiki/index.php?title=TUDelft/30_October_2008&action=edit 30] | [http://2008.igem.org/wiki/index.php?title=TUDelft/31_October_2008&action=edit 31] | ||
Contents |
September 4th
Transformation
3A Assembly
Though unknown whether ligation worked, we tried to transform cells with total volume (15 ul) of our ligation. Plated on Chloroamphenicol o/n @ 37ºC.
PCR
A new PCR with the Pfx enzyme was performed on the E.coli genes. This time more template DNA was used as the last times this PCR was performed it yielded no results. Two reactions of 50 ul were set up as last time. 50ng of template (genomic E. coli DNA) was used, other characteristics were the same.
Strangely, only one of the reactions yielded a result, for the idi gene. The picture of the gel with band cut out can be seen above. At this point, it is not clear what went wrong.
Double restriction
We did a double restriction (EcoRI & PstI) on the newly received living stabs, and ran a 2% agarose gel, because there were many parts < 200 bp. The gel is displayed in figure 1.
Most parts look all right. Due to the small parts having low mass and thus low light emittance, we used a lot of DNA, causing overexposure for the backbones. Only parts B0021 and F2620, a terminator and promoter, showed a smear in both cut and uncut DNA. B0021 was indeterminable, F2620 was correct size (which was also visible on the gel of the 2nd of September which was not posted). All the other parts were also likely to be correct size.
 "
"