Team:Paris/July 28
From 2008.igem.org
(→Results of the extraction : Electrophoresis) |
(→Results of the extraction : Electrophoresis) |
||
| (43 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
| - | == | + | == DNA Extraction == |
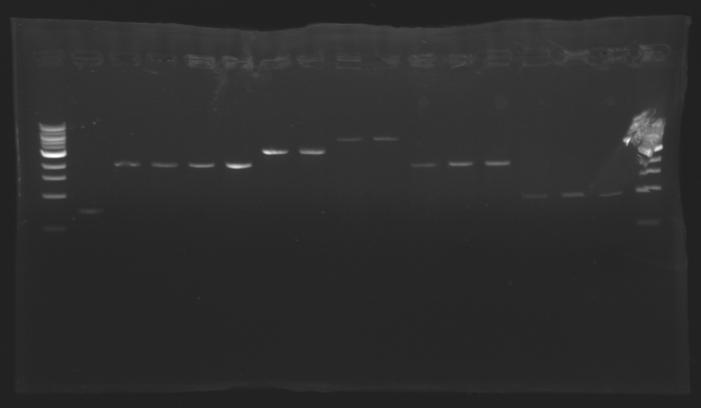
=== Results of the extraction : Electrophoresis === | === Results of the extraction : Electrophoresis === | ||
| - | conditions : | + | '''conditions of electrophoresis: ''' |
| + | |||
* 10µl of ladder 1 kb | * 10µl of ladder 1 kb | ||
* xµl of extraction added with 2µl of loading Dye 6x | * xµl of extraction added with 2µl of loading Dye 6x | ||
| Line 15: | Line 16: | ||
* 2µl of extraction added with 2µl of loading Dye 6x | * 2µl of extraction added with 2µl of loading Dye 6x | ||
| - | * Gel 5, 6 = 0,8% | + | * Gel 5, 6 = '''0,8%''' |
* 3µl of extraction added with 2µl of loading Dye 6x | * 3µl of extraction added with 2µl of loading Dye 6x | ||
| Line 25: | Line 26: | ||
| + | '''Results : ''' | ||
gel1 [[Image:080728gel_1.jpg| gel1|100px]] | gel1 [[Image:080728gel_1.jpg| gel1|100px]] | ||
| Line 59: | Line 61: | ||
! rowspan="2"| PstI | ! rowspan="2"| PstI | ||
! rowspan="2"| BI | ! rowspan="2"| BI | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 34 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| 8 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D101 | ! rowspan="1" style="background: #ccccff;" | D101 | ||
| Line 77: | Line 79: | ||
|align=center| FV | |align=center| FV | ||
|align=center| 2076 pb | |align=center| 2076 pb | ||
| - | |align=center| - | + | |align=center| - & 2000 pb |
| - | |align=center| - | + | |align=center| - & 7 |
| - | ! rowspan="1"| 1 | + | ! rowspan="1"| 1 & 7 |
| - | |align=center| 2 | + | |align=center| 2 & 2 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;" | D102 | ! rowspan="2" style="background: #ccccff;" | D102 | ||
| Line 89: | Line 91: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2077 pb | ! rowspan="2"| 2077 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 2000 pb |
| - | ! rowspan="1"| - | + | ! rowspan="1"| - & 5 |
| - | |align=center| 1 | + | |align=center| 1 & 7 |
| - | |align=center| 3 | + | |align=center| 3 & 3 |
|- | |- | ||
|align=center|5 | |align=center|5 | ||
| - | |align=center| - | + | |align=center| - & 10 |
| - | |align=center| 1 | + | |align=center| 1 & 7 |
| - | ! rowspan="1"| 4 | + | ! rowspan="1"| 4 & 4 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;" | D103 | ! rowspan="2" style="background: #ccccff;" | D103 | ||
| Line 106: | Line 108: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2100 pb | ! rowspan="2"| 2100 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 2000 pb |
! rowspan="1"| - | ! rowspan="1"| - | ||
|align=center| 1 | |align=center| 1 | ||
| Line 112: | Line 114: | ||
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | |align=center| | + | |align=center| 10 |
|align=center| 1 | |align=center| 1 | ||
! rowspan="1"| 6 | ! rowspan="1"| 6 | ||
| Line 123: | Line 125: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2100 pb | ! rowspan="2"| 2100 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 2000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 20 |
| - | ! rowspan="1"| 1 | + | ! rowspan="1"| 1 |
| - | ! rowspan="1"| 7 | + | ! rowspan="1"| 7 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 20 |
| - | ! rowspan="1"| 1 | + | ! rowspan="1"| 1 |
| - | ! rowspan="1"| 8 | + | ! rowspan="1"| 8 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;" | D105 | ! rowspan="2" style="background: #ccccff;" | D105 | ||
| Line 140: | Line 142: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2222 pb | ! rowspan="2"| 2222 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 2000 pb |
| - | |align=center| | + | |align=center| 5 |
| - | ! rowspan="1"| 2 | + | ! rowspan="1"| 2 |
| - | |align=center| 2 | + | |align=center| 2 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | |align=center| | + | |align=center| 5 |
| - | |align=center| 2 | + | |align=center| 2 |
! rowspan="1"| 3 | ! rowspan="1"| 3 | ||
|- | |- | ||
| Line 157: | Line 159: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2119 pb | ! rowspan="2"| 2119 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| - & 2000 pb |
| - | |align=center| | + | |align=center| - & 5 |
| - | ! rowspan="1"| 2 | + | ! rowspan="1"| 2 & 7 |
| - | |align=center| 4 | + | |align=center| 4 & 5 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | |align=center| | + | |align=center| - & 8 |
| - | |align=center| 2 | + | |align=center| 2 & 7 |
| - | ! rowspan="1"| 5 | + | ! rowspan="1"| 5 & 6 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D107 | ! rowspan="1" style="background: #ccccff;" | D107 | ||
| Line 174: | Line 176: | ||
! rowspan="1"| BV | ! rowspan="1"| BV | ||
! rowspan="1"| 2750 pb | ! rowspan="1"| 2750 pb | ||
| - | |align=center| | + | |align=center| - & 3000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| - & 5 |
| - | |align=center| 2 | + | |align=center| 2 & 7 |
| - | |align=center| 6 | + | |align=center| 6 & 7 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;" | D108 | ! rowspan="2" style="background: #ccccff;" | D108 | ||
| Line 186: | Line 188: | ||
! rowspan="2"| BI | ! rowspan="2"| BI | ||
! rowspan="2"| 707 pb | ! rowspan="2"| 707 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| - & - |
| - | |align=center| | + | |align=center| - & - |
| - | |align=center| 2 | + | |align=center| 2 & 7 |
| - | ! rowspan="1"| 7 | + | ! rowspan="1"| 7 & 8 |
|- | |- | ||
|align=center|3 | |align=center|3 | ||
| - | |align=center| | + | |align=center| - & - |
| - | |align=center| 2 | + | |align=center| 2 & 7 |
| - | ! rowspan="1"| 8 | + | ! rowspan="1"| 8 & 9 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D109 | ! rowspan="1" style="background: #ccccff;" | D109 | ||
| Line 203: | Line 205: | ||
|align=center|FI | |align=center|FI | ||
|align=center| 708 pb | |align=center| 708 pb | ||
| - | |align=center| | + | |align=center| - & - |
| - | |align=center| | + | |align=center| - & - |
| - | ! rowspan="1"| 3 | + | ! rowspan="1"| 3 & 7 |
| - | |align=center| 2 | + | |align=center| 2 & 10 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D110 | ! rowspan="1" style="background: #ccccff;" | D110 | ||
| Line 215: | Line 217: | ||
|align=center|BV | |align=center|BV | ||
|align=center| 2768 pb | |align=center| 2768 pb | ||
| - | |align=center| | + | |align=center| 3000 pb |
| - | |align=center| | + | |align=center| 20 |
|align=center| 3 | |align=center| 3 | ||
! rowspan="1"| 3 | ! rowspan="1"| 3 | ||
| Line 227: | Line 229: | ||
! rowspan="2"| BI | ! rowspan="2"| BI | ||
! rowspan="2"| 725 pb | ! rowspan="2"| 725 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| - |
| - | |align=center| | + | |align=center| - |
|align=center| 3 | |align=center| 3 | ||
! rowspan="1"| 4 | ! rowspan="1"| 4 | ||
|- | |- | ||
|align=center|3 | |align=center|3 | ||
| - | |align=center| | + | |align=center| - |
|align=center| 3 | |align=center| 3 | ||
! rowspan="1"| 5 | ! rowspan="1"| 5 | ||
| Line 244: | Line 246: | ||
|align=center|FI | |align=center|FI | ||
|align=center| 726 pb | |align=center| 726 pb | ||
| - | |align=center| | + | |align=center| - |
| - | |align=center| | + | |align=center| - |
! rowspan="1"| 3 | ! rowspan="1"| 3 | ||
|align=center| 6 | |align=center| 6 | ||
| Line 256: | Line 258: | ||
|align=center|FI | |align=center|FI | ||
|align=center| 779 pb | |align=center| 779 pb | ||
| - | |align=center| | + | |align=center| 800 pb |
| - | |align=center| | + | |align=center| 20 |
! rowspan="1"| 3 | ! rowspan="1"| 3 | ||
|align=center| 7 | |align=center| 7 | ||
| Line 268: | Line 270: | ||
|align=center|BI | |align=center|BI | ||
|align=center| 778 pb | |align=center| 778 pb | ||
| - | |align=center| | + | |align=center| 800 pb |
| - | |align=center| | + | |align=center| 20 |
|align=center| 3 | |align=center| 3 | ||
! rowspan="1"| 8 | ! rowspan="1"| 8 | ||
| Line 280: | Line 282: | ||
|align=center| FI | |align=center| FI | ||
|align=center| 746 pb | |align=center| 746 pb | ||
| - | |align=center| | + | |align=center| - & - |
| - | |align=center| | + | |align=center| - & - |
| - | |align=center| | + | |align=center| 4 & 7 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 2 & 11 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D116 | ! rowspan="1" style="background: #ccccff;" | D116 | ||
| Line 292: | Line 294: | ||
|align=center| BI | |align=center| BI | ||
|align=center| 745 pb | |align=center| 745 pb | ||
| - | |align=center| | + | |align=center| - & - |
| - | |align=center| | + | |align=center| - & - |
| - | ! rowspan="1"| | + | ! rowspan="1"| 4 & 7 |
| - | |align=center| | + | |align=center| 3 & 12 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;"|D117 | ! rowspan="1" style="background: #ccccff;"|D117 | ||
| Line 304: | Line 306: | ||
|align=center|FI | |align=center|FI | ||
|align=center| 746 pb | |align=center| 746 pb | ||
| - | |align=center| | + | |align=center| 700 pb |
| - | |align=center| | + | |align=center| 5 |
| - | |align=center| | + | |align=center| 4 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 4 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D118 | ! rowspan="1" style="background: #ccccff;" | D118 | ||
| Line 316: | Line 318: | ||
|align=center| BI | |align=center| BI | ||
|align=center| 745 pb | |align=center| 745 pb | ||
| - | |align=center| | + | |align=center| 700 pb |
| - | |align=center| | + | |align=center| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 4 |
| - | |align=center| | + | |align=center| 5 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;"|D119 | ! rowspan="1" style="background: #ccccff;"|D119 | ||
| Line 328: | Line 330: | ||
|align=center| FI | |align=center| FI | ||
|align=center| 743 pb | |align=center| 743 pb | ||
| - | |align=center| | + | |align=center| - & 800 pb |
| - | |align=center| | + | |align=center| - & 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 4 & 7 |
| - | |align=center| | + | |align=center| 6 & 13 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D120 | ! rowspan="1" style="background: #ccccff;" | D120 | ||
| Line 340: | Line 342: | ||
|align=center| BI | |align=center| BI | ||
|align=center| 742 pb | |align=center| 742 pb | ||
| - | |align=center| | + | |align=center| - & 800 pb |
| - | |align=center| | + | |align=center| - & 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 4 & 7 |
| - | |align=center| | + | |align=center| 7 & 14 |
|- | |- | ||
! rowspan="1" style="background: #ccccff;"|D121 | ! rowspan="1" style="background: #ccccff;"|D121 | ||
| Line 352: | Line 354: | ||
|align=center| FI | |align=center| FI | ||
|align=center| 704 pb | |align=center| 704 pb | ||
| - | |align=center| | + | |align=center| 700 pb |
| - | |align=center| | + | |align=center| 5 |
| - | |align=center| | + | |align=center| 4 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 8 |
|- | |- | ||
|- | |- | ||
| Line 365: | Line 367: | ||
|align=center| BI | |align=center| BI | ||
|align=center| 703 pb | |align=center| 703 pb | ||
| - | |align=center| | + | |align=center| 800 pb |
| - | |align=center| | + | |align=center| 10 |
| - | |align=center| | + | |align=center| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 2 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;"|D123 | ! rowspan="2" style="background: #ccccff;"|D123 | ||
| Line 377: | Line 379: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2100 pb | ! rowspan="2"| 2100 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 2000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 3 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 4 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;"|D124 | ! rowspan="2" style="background: #ccccff;"|D124 | ||
| Line 394: | Line 396: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2100 pb | ! rowspan="2"| 2100 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 2000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 15 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 6 |
|- | |- | ||
| - | ! rowspan="2" style="background: #ccccff;"| | + | ! rowspan="2" style="background: #ccccff;"|D125 |
| - | ! rowspan="2" style="background: #ccccff;"| | + | ! rowspan="2" style="background: #ccccff;"|B0015 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"|EcoRI | ! rowspan="2"|EcoRI | ||
| Line 411: | Line 413: | ||
! rowspan="2"| FV | ! rowspan="2"| FV | ||
! rowspan="2"| 3303 pb | ! rowspan="2"| 3303 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 3000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 15 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 7 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 15 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 8 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;"|D126 | ! rowspan="2" style="background: #ccccff;"|D126 | ||
| Line 428: | Line 430: | ||
! rowspan="2"| FV | ! rowspan="2"| FV | ||
! rowspan="2"| 5621 pb | ! rowspan="2"| 5621 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 6000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 9 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;" | D127 | ! rowspan="2" style="background: #ccccff;" | D127 | ||
| Line 445: | Line 447: | ||
! rowspan="2"| BI | ! rowspan="2"| BI | ||
! rowspan="2"| 37 pb | ! rowspan="2"| 37 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| - & - |
| - | ! rowspan="1"| | + | ! rowspan="1"| - & - |
| - | ! rowspan="1"| | + | ! rowspan="1"| 8 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 3 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
|- | |- | ||
! rowspan="1" style="background: #ccccff;" | D128 | ! rowspan="1" style="background: #ccccff;" | D128 | ||
| Line 462: | Line 464: | ||
! rowspan="1"| FV | ! rowspan="1"| FV | ||
! rowspan="1"| 2079 pb | ! rowspan="1"| 2079 pb | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 2000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 11 |
|- | |- | ||
! rowspan="2" style="background: #ccccff;" | D129 | ! rowspan="2" style="background: #ccccff;" | D129 | ||
| Line 474: | Line 476: | ||
! rowspan="2"| BV | ! rowspan="2"| BV | ||
! rowspan="2"| 2080 pb | ! rowspan="2"| 2080 pb | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| 2000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 12 |
|- | |- | ||
|align=center|5 | |align=center|5 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 13 |
|- | |- | ||
! rowspan="3" style="background: #ccccff;"|D130 | ! rowspan="3" style="background: #ccccff;"|D130 | ||
| Line 491: | Line 493: | ||
! rowspan="3"| BI | ! rowspan="3"| BI | ||
! rowspan="3"| 939 pb | ! rowspan="3"| 939 pb | ||
| - | ! rowspan="3"| | + | ! rowspan="3"| 1100 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 14 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 15 |
|- | |- | ||
|align=center|3 | |align=center|3 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 16 |
|- | |- | ||
! rowspan="3" style="background: #ccccff;"|D131 | ! rowspan="3" style="background: #ccccff;"|D131 | ||
| Line 513: | Line 515: | ||
! rowspan="3"| BI | ! rowspan="3"| BI | ||
! rowspan="3"| 900 pb | ! rowspan="3"| 900 pb | ||
| - | ! rowspan=" | + | ! rowspan="3"| 1000 pb |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 6 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 3 |
|- | |- | ||
|align=center|2 | |align=center|2 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 6 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 4 |
|- | |- | ||
|align=center|3 | |align=center|3 | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 6 |
| - | ! rowspan="1"| | + | ! rowspan="1"| 5 |
|} | |} | ||
| - | ==> | + | ==> ''Conclusion :'' for most of the samples we have enough signal to determine the concentration of the digestion to do the ligation. |
| + | |||
| + | |||
| + | For the samples we don't succeed to obtain signals, we have migrated a second gel ( gel n°7). | ||
| + | |||
| + | |||
| + | ==> ''Conclusion :'' The gel n°7 allowed us to know the concentration of other digestion undetermined. | ||
| + | |||
| + | == Ligation == | ||
| + | |||
| + | |||
| + | === Protocol === | ||
| + | |||
| + | For each samples, | ||
| + | |||
| + | * 1 µl Ligase | ||
| + | * X µl Vector | ||
| + | * Y µl Insert (3x more than vector) | ||
| + | * 2 µl Ligase Buffer 10x | ||
| + | * 20 µl qsp H2O | ||
| + | |||
| + | * Incubates O/N at 4°C (in the fridge) | ||
| + | |||
| + | |||
| + | === List of ligations === | ||
| + | |||
| + | {| border="1" | ||
| + | |align=center|'''Name''' | ||
| + | |align=center|'''Vector''' | ||
| + | |align=center|'''Insert''' | ||
| + | |align=center|'''Antibio''' | ||
| + | |align=center|'''Vol. vector (µl)''' | ||
| + | |align=center|'''Vol. insert (µl)''' | ||
| + | |align=center|'''Vol. H20 (µl)''' | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L100 | ||
| + | |align=center| D110 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|3 | ||
| + | |align=center|6 | ||
| + | |align=center|8 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L101 | ||
| + | |align=center| D110 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|3 | ||
| + | |align=center|6 | ||
| + | |align=center|8 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L102 | ||
| + | |align=center| D129 | ||
| + | |align=center| D118 | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|10 | ||
| + | |align=center|2 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L103 | ||
| + | |align=center| D129 | ||
| + | |align=center| D122 | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|5 | ||
| + | |align=center|7 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L104 | ||
| + | |align=center| D129 | ||
| + | |align=center| D114 | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|4 | ||
| + | |align=center|8 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L105 | ||
| + | |align=center| D123 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|4 | ||
| + | |align=center|8 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L106 | ||
| + | |align=center| D123 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|8 | ||
| + | |align=center|4 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L107 | ||
| + | |align=center| D103 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|8 | ||
| + | |align=center|4 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L108 | ||
| + | |align=center| D103 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|8 | ||
| + | |align=center|4 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L109 | ||
| + | |align=center| D124 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|4 | ||
| + | |align=center|7 | ||
| + | |align=center|6 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L110 | ||
| + | |align=center| D124 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|4 | ||
| + | |align=center|7 | ||
| + | |align=center|6 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L111 | ||
| + | |align=center| D104 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|3 | ||
| + | |align=center|5 | ||
| + | |align=center|9 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L112 | ||
| + | |align=center| D104 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|3 | ||
| + | |align=center|5 | ||
| + | |align=center|9 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L113 | ||
| + | |align=center| D126 | ||
| + | |align=center| D130 | ||
| + | |align=center| K | ||
| + | |align=center|5 | ||
| + | |align=center|2.5 | ||
| + | |align=center|9.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L114 | ||
| + | |align=center| D126 | ||
| + | |align=center| D131 | ||
| + | |align=center| K | ||
| + | |align=center|5 | ||
| + | |align=center|2.5 | ||
| + | |align=center|9.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L115 | ||
| + | |align=center| D105 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|7 | ||
| + | |align=center|11 | ||
| + | |align=center|- | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L116 | ||
| + | |align=center| D105 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|7 | ||
| + | |align=center|11 | ||
| + | |align=center|- | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L117 | ||
| + | |align=center| D125 | ||
| + | |align=center| D117 | ||
| + | |align=center| A | ||
| + | |align=center|4 | ||
| + | |align=center|3 | ||
| + | |align=center|10 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L118 | ||
| + | |align=center| D125 | ||
| + | |align=center| D121 | ||
| + | |align=center| A | ||
| + | |align=center|4 | ||
| + | |align=center|2 | ||
| + | |align=center|11 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L119 | ||
| + | |align=center| D125 | ||
| + | |align=center| D113 | ||
| + | |align=center| A | ||
| + | |align=center|4 | ||
| + | |align=center|2 | ||
| + | |align=center|11 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L120 | ||
| + | |align=center| D106 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|6 | ||
| + | |align=center|7.5 | ||
| + | |align=center|3.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L121 | ||
| + | |align=center| D106 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|6 | ||
| + | |align=center|7.5 | ||
| + | |align=center|3.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L122 | ||
| + | |align=center| D107 | ||
| + | |align=center| D130 | ||
| + | |align=center| A | ||
| + | |align=center|7 | ||
| + | |align=center|3.5 | ||
| + | |align=center|6.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L123 | ||
| + | |align=center| D107 | ||
| + | |align=center| D131 | ||
| + | |align=center| A | ||
| + | |align=center|7 | ||
| + | |align=center|3.5 | ||
| + | |align=center|6.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L124 | ||
| + | |align=center| D102 | ||
| + | |align=center| D122 | ||
| + | |align=center| A | ||
| + | |align=center|6 | ||
| + | |align=center|5 | ||
| + | |align=center|4 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L125 | ||
| + | |align=center| D102 | ||
| + | |align=center| D1118 | ||
| + | |align=center| A | ||
| + | |align=center|6 | ||
| + | |align=center|10 | ||
| + | |align=center|1 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L126 | ||
| + | |align=center| D102 | ||
| + | |align=center| D114 | ||
| + | |align=center| A | ||
| + | |align=center|6 | ||
| + | |align=center|4 | ||
| + | |align=center|7 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| L127 | ||
| + | |align=center| D125 | ||
| + | |align=center| D119 | ||
| + | |align=center| A | ||
| + | |align=center|6 | ||
| + | |align=center|3 | ||
| + | |align=center|8 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C1 | ||
| + | |align=center| D110 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|3 | ||
| + | |align=center|- | ||
| + | |align=center|14 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C2 | ||
| + | |align=center| D129 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|- | ||
| + | |align=center|12 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C3 | ||
| + | |align=center| D123 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|5 | ||
| + | |align=center|- | ||
| + | |align=center|12 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C4 | ||
| + | |align=center| D103 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|8 | ||
| + | |align=center|- | ||
| + | |align=center|9 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C5 | ||
| + | |align=center| D124 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|7 | ||
| + | |align=center|- | ||
| + | |align=center|10 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C6 | ||
| + | |align=center| D104 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|3 | ||
| + | |align=center|- | ||
| + | |align=center|14 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C7 | ||
| + | |align=center| D126 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|2.5 | ||
| + | |align=center|- | ||
| + | |align=center|14.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C8 | ||
| + | |align=center| D105 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|11 | ||
| + | |align=center|- | ||
| + | |align=center|6 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C9 | ||
| + | |align=center| D125 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|2 | ||
| + | |align=center|- | ||
| + | |align=center|15 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C10 | ||
| + | |align=center| D106 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|7.5 | ||
| + | |align=center|- | ||
| + | |align=center|9.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C11 | ||
| + | |align=center| D107 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|3.5 | ||
| + | |align=center|- | ||
| + | |align=center|13.5 | ||
| + | |- | ||
| + | |style="background: #ccccff;"| C12 | ||
| + | |align=center| D102 | ||
| + | |align=center| - | ||
| + | |align=center| A | ||
| + | |align=center|10 | ||
| + | |align=center|- | ||
| + | |align=center|7 | ||
| + | |} | ||
| + | |||
| + | == Purification of the B0034 and B0030 BioBricks == | ||
| + | |||
| + | ''We performed PCR on the B0034 BioBrick (MP 100) and the B0030 BioBrick (MP 120) to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.'' | ||
| + | |||
| + | === PCR Protocol === | ||
| + | |||
| + | ''For each samples,'' | ||
| + | |||
| + | * 1 µl dNTP | ||
| + | * 10 µl Buffer Phusion 5x | ||
| + | * 2,5 µl VR2 (O18) | ||
| + | * 2,5 µl VF (O19) | ||
| + | * 1 µl Phusion | ||
| + | * 50 µl qsp H2O (33µl) | ||
| + | |||
| + | |||
| + | === Electrophoresis - Purification === | ||
| + | |||
| + | ''When the PCR cycles were finished,'' | ||
| + | |||
| + | * Add 10 µL of 6X loading dye. | ||
| + | * Load the samples (2 x 30 µL per sample) on a 1,5% agarose gel. | ||
| + | * Migration 30' at 100W. | ||
| + | |||
| + | |||
| + | ''After electrophoresis,'' | ||
| + | |||
| + | * Excision and purification of the bands corresponding to MP 100 and MP 120, using the QIAquick DNA Gel Extraction Kit (QIAGEN). | ||
| + | * Elution in 50 µL of water. | ||
| + | |||
| + | ''Because the intensity of the band corresponding to MP 120 was very low, we only continued with MP 100.'' | ||
| + | |||
| + | |||
| + | === DNA Digestion === | ||
| + | |||
| + | ''MP 100 was digested by EcoRI & SpeI (Forward Insert) or by XbaI & PstI (D100 : Backward Insert) | ||
| + | |||
| + | Digestion reaction (total volume : 50 µL)'' | ||
| + | |||
| + | * 25 µL DNA | ||
| + | * 5 µL buffer 2 (10X) | ||
| + | * 2 µL enzyme 1 | ||
| + | * 2 µL enzyme 2 | ||
| + | * 0.5 µL BSA (100X) | ||
| + | * 15,5 µL water | ||
| + | |||
| + | * The reaction was incubated 2 hours at 37°C. | ||
| + | * The samples (2 x 30 µL per sample) were then analysed by electrophoresis. | ||
| + | |||
| + | |||
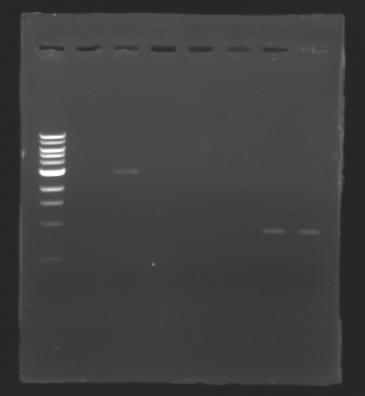
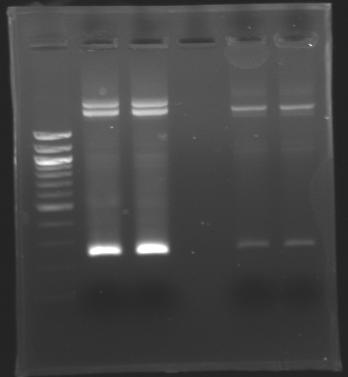
| + | ===Electrophoresis=== | ||
| + | |||
| + | |||
| + | |||
| + | {| border="1" | ||
| + | |align=center|'''1''' | ||
| + | |align=center|'''2''' | ||
| + | |align=center|'''3''' | ||
| + | |align=center|'''4''' | ||
| + | |align=center|'''5''' | ||
| + | |align=center|'''6''' | ||
| + | |- | ||
| + | ! rowspan="1"|ladder | ||
| + | ! rowspan="1"|EcoRI & SpeI | ||
| + | ! rowspan="1"|EcoRI & SpeI | ||
| + | ! rowspan="1"| - | ||
| + | ! rowspan="1"|XbaI & PstI | ||
| + | ! rowspan="1"|XbaI & PstI | ||
| + | |} | ||
| + | |||
| + | [[Image:Image_gel.jpg|thumb|]] | ||
| + | |||
| + | The sequence of MP 100 (B0034) digested by EcoRI & SpeI (35 bp) or XbaI & PstI (34 bp) was too short and we didn't manage to visualise it on the gel. | ||
| + | |||
| + | ==> ''Conclusion'' : small parts like B0034 can't be cloned as an insert. | ||
Latest revision as of 16:59, 13 August 2008
|
DNA ExtractionResults of the extraction : Electrophoresisconditions of electrophoresis:
LigationProtocolFor each samples,
List of ligations
Purification of the B0034 and B0030 BioBricksWe performed PCR on the B0034 BioBrick (MP 100) and the B0030 BioBrick (MP 120) to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments. PCR ProtocolFor each samples,
Electrophoresis - PurificationWhen the PCR cycles were finished,
Because the intensity of the band corresponding to MP 120 was very low, we only continued with MP 100.
DNA DigestionMP 100 was digested by EcoRI & SpeI (Forward Insert) or by XbaI & PstI (D100 : Backward Insert) Digestion reaction (total volume : 50 µL)
Electrophoresis
The sequence of MP 100 (B0034) digested by EcoRI & SpeI (35 bp) or XbaI & PstI (34 bp) was too short and we didn't manage to visualise it on the gel. ==> Conclusion : small parts like B0034 can't be cloned as an insert. |
 "
"