Team:Paris/July 29
From 2008.igem.org
(Difference between revisions)
(→List of the Ligation Transformation) |
|||
| (72 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{Paris/Calendar_Links|July | + | {{Paris/Calendar_Links|July 28|July 30}} |
| - | == | + | ==DNA digestion and purification== |
| - | for each reaction (total volume : 50 µL) | + | |
| + | === Mix digestion === | ||
| + | |||
| + | ''for each reaction (total volume : 50 µL)'' | ||
* 20 µL of DNA (MiniPrep product) | * 20 µL of DNA (MiniPrep product) | ||
* 2 µL of enzyme 1 | * 2 µL of enzyme 1 | ||
* 2 µL of enzyme 2 | * 2 µL of enzyme 2 | ||
* 5 µL of buffer 2 (10X) | * 5 µL of buffer 2 (10X) | ||
| + | * 0,5 µL of BSA | ||
* 20,5 µL water | * 20,5 µL water | ||
| - | Each reaction was | + | |
| + | |||
| + | === Protocol === | ||
| + | |||
| + | Each reaction was : | ||
| + | * Incubate 2 hours at 37°C, then 10 minutes at 60-65°C (to inactivate the enzymes). | ||
| + | * Add 10 µL of loading dye (6X) to each of the 50 µL of digestion product. | ||
| + | * Run the whole samples in a '''1,5% agarose gel''' (about 30 minutes at 100 W ; 2 x 30 µL per sample ; 30 µL per well). | ||
| + | * Excise the bands of interest from the gel and the DNA was purified using the QIAquick DNA Gel Extraction kit (QIAGEN). | ||
| + | * The elution of DNA was performed using 50 µL of water (after 10 minutes of incubation at 37°C). | ||
| + | |||
| + | ==> Unfortunately, there were not enough columms, so we took some columms from the QIAGEN MiniPrep kit, hoping that it will work with the QIAquick DNA Gel Extraction kit. Some of the samples were too voluminous, so we separated them into two tubes. | ||
| + | |||
| + | |||
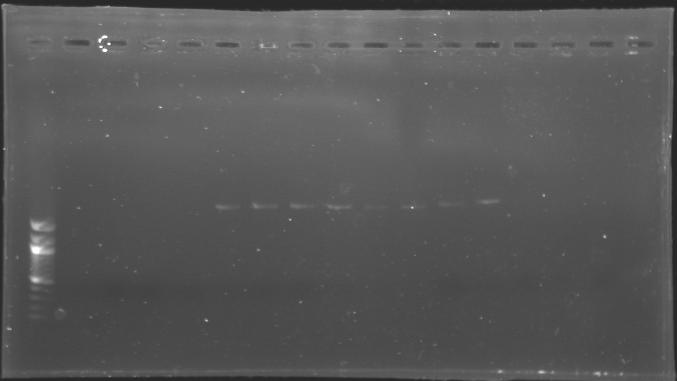
Each of the samples was then analysed by a 1,5% agarose gel: | Each of the samples was then analysed by a 1,5% agarose gel: | ||
| Line 18: | Line 35: | ||
The ladder used was the 100 bp ladder from New England Biolabs. | The ladder used was the 100 bp ladder from New England Biolabs. | ||
| - | + | ||
| + | |||
| + | === List of the digestion === | ||
| + | |||
| + | [[Image:KR000081.jpg|thumb|]] | ||
{| border="1" | {| border="1" | ||
| Line 30: | Line 51: | ||
|align=center|'''Mea Size ''' | |align=center|'''Mea Size ''' | ||
|align=center|'''Conc (ng/µl)''' | |align=center|'''Conc (ng/µl)''' | ||
| - | |||
|align=center|'''Band''' | |align=center|'''Band''' | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | D101 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | B0034 |
| - | + | ! rowspan="1"|1 | |
| - | ! rowspan=" | + | ! rowspan="1"| EcoRI |
| - | ! rowspan=" | + | ! rowspan="1"| XbaI |
| - | ! rowspan=" | + | ! rowspan="1"| FV |
| - | ! rowspan=" | + | ! rowspan="1"| 2076 pb |
! rowspan="1"| - | ! rowspan="1"| - | ||
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| 10 |
| - | + | ||
|- | |- | ||
| - | | | + | ! rowspan="1" style="background: #ccccff;" | D102 |
| + | ! rowspan="1" style="background: #ccccff;" | B0034 | ||
| + | ! rowspan="1"|1 | ||
| + | ! rowspan="1"| SpeI | ||
| + | ! rowspan="1"| PstI | ||
| + | ! rowspan="1"| BV | ||
| + | ! rowspan="1"| 2077 pb | ||
! rowspan="1"| - | ! rowspan="1"| - | ||
! rowspan="1"| - | ! rowspan="1"| - | ||
| + | ! rowspan="1"| 11 | ||
| + | |- | ||
| + | ! rowspan="1" style="background: #ccccff;" | D105 | ||
| + | ! rowspan="1" style="background: #ccccff;" | R0079 | ||
| + | ! rowspan="1"|1 | ||
| + | ! rowspan="1"| SpeI | ||
| + | ! rowspan="1"| PstI | ||
| + | ! rowspan="1"| BV | ||
| + | ! rowspan="1"| 2222 pb | ||
! rowspan="1"| - | ! rowspan="1"| - | ||
| + | ! rowspan="1"| - | ||
| + | ! rowspan="1"| 13 | ||
|- | |- | ||
| - | ! rowspan="1" style="background: #ccccff;" | | + | ! rowspan="1" style="background: #ccccff;" | D106 |
| - | ! rowspan="1" style="background: #ccccff;" | | + | ! rowspan="1" style="background: #ccccff;" | R0040 |
| - | + | ! rowspan="1"|1 | |
| - | + | ! rowspan="1"| SpeI | |
| - | + | ! rowspan="1"| PstI | |
| - | + | ! rowspan="1"| BV | |
| - | + | ! rowspan="1"| 2119 pb | |
| - | + | ! rowspan="1"| - | |
| - | + | ! rowspan="1"| - | |
| - | ! rowspan="1"| | + | ! rowspan="1"| 12 |
| - | + | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | D108 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | S03154 |
| - | + | ! rowspan="1"|1 | |
| - | ! rowspan=" | + | ! rowspan="1"| XbaI |
| - | ! rowspan=" | + | ! rowspan="1"| PstI |
| - | ! rowspan=" | + | ! rowspan="1"| BI |
| - | ! rowspan=" | + | ! rowspan="1"| 707 pb |
| - | ! rowspan=" | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"| - |
| - | + | ! rowspan="1"| 14 | |
| - | + | ||
|- | |- | ||
| - | | | + | ! rowspan="1" style="background: #ccccff;" | D111 |
| - | | | + | ! rowspan="1" style="background: #ccccff;" | S03879 |
| - | | | + | ! rowspan="1"|1 |
| - | ! rowspan="1"| | + | ! rowspan="1"| XbaI |
| + | ! rowspan="1"| PstI | ||
| + | ! rowspan="1"| BI | ||
| + | ! rowspan="1"| 725 pb | ||
| + | ! rowspan="1"| - | ||
| + | ! rowspan="1"|- | ||
| + | ! rowspan="1"| 15 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | D115 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | C0179 |
| - | + | ! rowspan="1"|2 | |
| - | ! rowspan=" | + | ! rowspan="1"| EcoRI |
| - | ! rowspan=" | + | ! rowspan="1"| SpeI |
| - | ! rowspan=" | + | ! rowspan="1"| FI |
| - | ! rowspan=" | + | ! rowspan="1"| 746 pb |
| - | ! rowspan=" | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"|- |
| - | + | ! rowspan="1"| 4 & 5 | |
| - | + | ||
|- | |- | ||
| - | | | + | ! rowspan="1" style="background: #ccccff;" | D116 |
| - | | | + | ! rowspan="1" style="background: #ccccff;" | C0179 |
| - | | | + | ! rowspan="1"|2 |
| - | ! rowspan="1"| | + | ! rowspan="1"| XbaI |
| + | ! rowspan="1"| PstI | ||
| + | ! rowspan="1"| BI | ||
| + | ! rowspan="1"| 745 pb | ||
| + | ! rowspan="1"|- | ||
| + | ! rowspan="1"| - | ||
| + | ! rowspan="1"| 2 & 3 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | D117 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | E0030 |
| - | + | ! rowspan="1"|1 | |
| - | ! rowspan=" | + | ! rowspan="1"| EcoRI |
| - | ! rowspan=" | + | ! rowspan="1"| SpeI |
| - | ! rowspan=" | + | ! rowspan="1"| FI |
| - | ! rowspan=" | + | ! rowspan="1"| 746 pb |
| - | ! rowspan=" | + | ! rowspan="1"| - |
| - | ! rowspan="1"| | + | ! rowspan="1"|- |
| - | ! rowspan="1"| | + | ! rowspan="1"| 16 |
| - | ! rowspan="1"| | + | |
|- | |- | ||
| - | | | + | ! rowspan="1" style="background: #ccccff;" | D128 |
| - | ! rowspan="1"| | + | ! rowspan="1" style="background: #ccccff;" | B0030 |
| - | ! rowspan="1"| 1 | + | ! rowspan="1"|2 |
| - | ! rowspan="1"| | + | ! rowspan="1"|EcoRI |
| + | ! rowspan="1"|XbaI | ||
| + | ! rowspan="1"|FV | ||
| + | ! rowspan="1"| 2079 pb | ||
| + | ! rowspan="1"| - | ||
| + | ! rowspan="1"|- | ||
| + | ! rowspan="1"| 6 & 7 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | D129 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #ccccff;" | B0030 |
| - | + | ! rowspan="1"|2 | |
| - | ! rowspan=" | + | ! rowspan="1"|SpeI |
| - | ! rowspan=" | + | ! rowspan="1"|PstI |
| - | ! rowspan=" | + | ! rowspan="1"|BV |
| - | ! rowspan=" | + | ! rowspan="1"| 2080 pb |
| - | ! rowspan=" | + | ! rowspan="1"| - |
| - | + | ! rowspan="1"|- | |
| - | ! rowspan="1"| 2 | + | ! rowspan="1"| 8 & 9 |
| - | |align=center| | + | |} |
| + | |||
| + | ==> '''Conclusion :'''The amount of DNA loaded was too low and the DNA ladder used was the wrong one. So this experiment has to be reconducted tomorrow. | ||
| + | |||
| + | == Transformations == | ||
| + | |||
| + | === Protocol === | ||
| + | |||
| + | ''Use of TOP10 Chemically competent cells'' | ||
| + | |||
| + | * Defroze competent cells on ice during 5' | ||
| + | * Add 5µl of DNA Ligation in 50µL of competent bacterias (or 1µL for the positive control puc19) | ||
| + | * Incubate 30' on ice | ||
| + | * Heat-shock the cells during 30" at 42°C without shaking | ||
| + | * Put 2' on ice | ||
| + | * Add 250µL of pre-warmed SOC medium (4°C) | ||
| + | * Incubate 1h at 37°C under shaking (225rpm) | ||
| + | * Spin at 5.000rpm during 30" | ||
| + | * Remove 150µL of supernatant | ||
| + | * Resuspent the pellet in the 150µL left | ||
| + | * Spread on adequated plates | ||
| + | * Incubate O/N at 37°C | ||
| + | |||
| + | |||
| + | === List of the Ligation Transformation === | ||
| + | |||
| + | |||
| + | {| border="1" | ||
| + | |align="center"|'''Name''' | ||
| + | |align="center"|'''Description''' | ||
| + | |align="center"|'''Antibio''' | ||
|- | |- | ||
| - | | | + | |colspan="3"|'''Ligation ''' |
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | + | |align="center"|L100 | |
| - | + | |align="center"|rbs TetR - ECFP<br>D110 (BV) - D130 (BI) | |
| - | |align=center | + | |align="center"|Amp |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |align=center| | + | |align="center"|L101 |
| - | |align=center| | + | |align="center"|rbs TetR - GFP tripart<br>D110 (BV) - D131 (BI) |
| - | |align | + | |align="center"|Amp |
| - | + | ||
|- | |- | ||
| - | + | |align="center"|L102 | |
| - | + | |align="center"|Strong rbs - YFP<br>D129 (BV) - D118 (BI) | |
| - | |align=center | + | |align="center"|Amp |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |align=center | + | |
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | + | |align="center"|L103 | |
| - | + | |align="center"|Strong rbs - mRFP<br>D129 (BV) - D122 (BI) | |
| - | |align=center | + | |align="center"|Amp |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |align=center| | + | |align="center"|L104 |
| - | |align=center| | + | |align="center"|Strong rbs - lasR activator<br>D129 (BV) - D114 (BI) |
| - | |align | + | |align="center"|Amp |
| - | + | ||
|- | |- | ||
| - | + | |align="center"|L105 | |
| - | + | |align="center"|Strong promoter - ECFP<br>D123 (BV) - D130 (BI) | |
| - | |align=center| | + | |align="center"|Amp |
| - | |align=center | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | + | |align="center"|L106 | |
| - | + | |align="center"|Strong promoter - gfp Tripart<br>D123 (BV) - D131 (BI) | |
| - | |align=center| | + | |align="center"|Amp |
| - | |align=center| | + | |- |
| - | |align=center| | + | |align="center"|L107 |
| - | |align=center|BV | + | |align="center"|Strongest promoter - ECFP<br>D103 (BV) - D130 (BI) |
| - | |align=center| | + | |align="center"|Amp |
| - | |align=center| | + | |- |
| - | |align=center| | + | |align="center"|L108 n°2 (the right one) |
| - | |align=center| | + | |align="center"|Strong promoter - gfp Tripart<br>D103 (BV) - D131 (BI) |
| - | + | |align="center"|Amp | |
| + | |- | ||
| + | |align="center"|L109 n°1 | ||
| + | |align="center"|Strong promoter - ecfp<br>D124 (BV) - D130 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L109 n°2 | ||
| + | |align="center"|Strong promoter - ecfp<br>D124 (BV) - D130 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L110 | ||
| + | |align="center"|Medium promoter - gfp Tripart<br>D124 (BV) - D131 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L111 | ||
| + | |align="center"|Weak promoter - ECFP<br>D104 (BV) - D130 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L112 | ||
| + | |align="center"|Weak promoter - gfp tripart<br>D104 (BV) - D131 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L113 | ||
| + | |align="center"|AracpBAD - ecfp<br>D126 (BV) - D130 (BI) | ||
| + | |align="center"|Kan | ||
| + | |- | ||
| + | |align="center"|L114 | ||
| + | |align="center"|AracpBAD - gfp tripart<br>D126 (BV) - D131 (BI) | ||
| + | |align="center"|Kan | ||
| + | |- | ||
| + | |align="center"|L115 | ||
| + | |align="center"|pLas - ECFP<br>D105 (BV) - D130 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L116 | ||
| + | |align="center"|pLas - gfp Tripart<br>D105 (BV) - D131 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L117 | ||
| + | |align="center"|yfp - Double Terminator<br>D117 (FI) - D125 (FV) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L118 | ||
| + | |align="center"|rfp - Double Terminator<br>D121 (FI) - D125 (FV) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L119 | ||
| + | |align="center"|lasR activator + LVA - Double Terminator<br>D113 (FI) - D125 (FV) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L120 | ||
| + | |align="center"|tetR repressible promoter - ECFP<br>D106 (BV) - D130 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L121 | ||
| + | |align="center"|Strong promoter - gfp tripart<br>D106 (BV) - D131 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L122 | ||
| + | |align="center"|RBS-lasI - ecfp<br>D107 (BV) - D130 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L123 | ||
| + | |align="center"|RBS lasI - gfp Tripart<br>D107 (BV) - D131 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L124 | ||
| + | |align="center"|Strongest RBS - mRFP<br>D102 (BV) - D122 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L125 | ||
| + | |align="center"|Strongest RBS - yfp<br>D102 (BV) - D118 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L126 | ||
| + | |align="center"| | ||
| + | Strongest RBS (1)- LacR activator (+LVA)<br>D102 (BV) - D114 (BI) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|L127 | ||
| + | |align="center"|gfp (1)- Double terminator<br>D119 (FI) - D125 (FV) | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |colspan ="3"|'''Controls''' | ||
| + | |- | ||
| + | |align="center"|C1 | ||
| + | |align="center"|D110 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C2 | ||
| + | |align="center"|D129 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C3 | ||
| + | |align="center"|D123 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C4 | ||
| + | |align="center"|D103 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C5 | ||
| + | |align="center"|D124 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C6 | ||
| + | |align="center"|D104 | ||
| + | |align="center"|Amp | ||
| + | |||
| + | |- | ||
| + | |align="center"|C7 | ||
| + | |align="center"|D126 | ||
| + | |align="center"|Kana | ||
| + | |- | ||
| + | |align="center"|C8 | ||
| + | |align="center"|D105 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C9 | ||
| + | |align="center"|D125 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C10 | ||
| + | |align="center"|D106 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C11 | ||
| + | |align="center"|D107 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|C12 | ||
| + | |align="center"|D102 | ||
| + | |align="center"|Amp | ||
| + | |- | ||
| + | |align="center"|Positive control | ||
| + | |align="center"|puc19 | ||
| + | |align="center"|Amp | ||
|} | |} | ||
Latest revision as of 17:01, 13 August 2008
DNA digestion and purificationMix digestionfor each reaction (total volume : 50 µL)
ProtocolEach reaction was :
==> Unfortunately, there were not enough columms, so we took some columms from the QIAGEN MiniPrep kit, hoping that it will work with the QIAquick DNA Gel Extraction kit. Some of the samples were too voluminous, so we separated them into two tubes.
Each of the samples was then analysed by a 1,5% agarose gel:
The ladder used was the 100 bp ladder from New England Biolabs.
List of the digestion
==> Conclusion :The amount of DNA loaded was too low and the DNA ladder used was the wrong one. So this experiment has to be reconducted tomorrow. TransformationsProtocolUse of TOP10 Chemically competent cells
List of the Ligation Transformation
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"