Team:Paris/July 28
From 2008.igem.org
(→Ligation) |
(→Results of the extraction : Electrophoresis) |
||
| (28 intermediate revisions not shown) | |||
| Line 406: | Line 406: | ||
! rowspan="1"| 6 | ! rowspan="1"| 6 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: #ccccff;"| | + | ! rowspan="2" style="background: #ccccff;"|D125 |
| - | ! rowspan="2" style="background: #ccccff;"| | + | ! rowspan="2" style="background: #ccccff;"|B0015 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"|EcoRI | ! rowspan="2"|EcoRI | ||
| Line 532: | Line 532: | ||
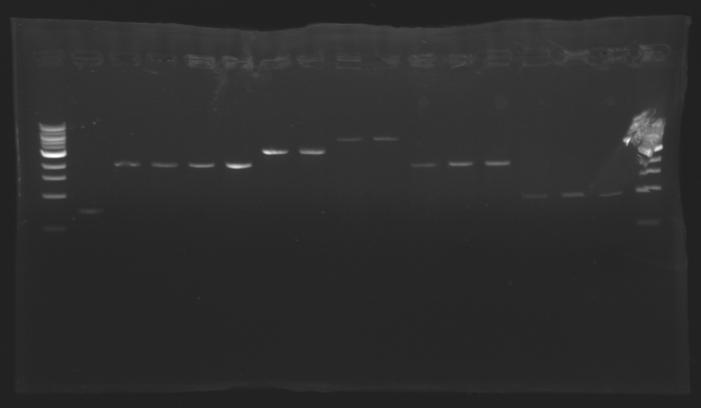
| - | ==> Conclusion : for most of the samples we have enough signal to determine the concentration of the digestion to do the ligation. | + | ==> ''Conclusion :'' for most of the samples we have enough signal to determine the concentration of the digestion to do the ligation. |
| Line 538: | Line 538: | ||
| - | ==> Conclusion : The gel n°7 allowed us to know the concentration of other digestion undetermined. | + | ==> ''Conclusion :'' The gel n°7 allowed us to know the concentration of other digestion undetermined. |
== Ligation == | == Ligation == | ||
| + | |||
=== Protocol === | === Protocol === | ||
| Line 551: | Line 552: | ||
* 2 µl Ligase Buffer 10x | * 2 µl Ligase Buffer 10x | ||
* 20 µl qsp H2O | * 20 µl qsp H2O | ||
| - | |||
* Incubates O/N at 4°C (in the fridge) | * Incubates O/N at 4°C (in the fridge) | ||
| + | === List of ligations === | ||
{| border="1" | {| border="1" | ||
| Line 566: | Line 567: | ||
|align=center|'''Vol. H20 (µl)''' | |align=center|'''Vol. H20 (µl)''' | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L100 |
|align=center| D110 | |align=center| D110 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 574: | Line 575: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L101 |
|align=center| D110 | |align=center| D110 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 582: | Line 583: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L102 |
|align=center| D129 | |align=center| D129 | ||
| - | |align=center| | + | |align=center| D118 |
|align=center| A | |align=center| A | ||
|align=center|5 | |align=center|5 | ||
| Line 590: | Line 591: | ||
|align=center|2 | |align=center|2 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L103 |
|align=center| D129 | |align=center| D129 | ||
|align=center| D122 | |align=center| D122 | ||
| Line 598: | Line 599: | ||
|align=center|7 | |align=center|7 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L104 |
|align=center| D129 | |align=center| D129 | ||
|align=center| D114 | |align=center| D114 | ||
| Line 606: | Line 607: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L105 |
|align=center| D123 | |align=center| D123 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 614: | Line 615: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L106 |
|align=center| D123 | |align=center| D123 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 622: | Line 623: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L107 |
|align=center| D103 | |align=center| D103 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 630: | Line 631: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L108 |
|align=center| D103 | |align=center| D103 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 638: | Line 639: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L109 |
|align=center| D124 | |align=center| D124 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 646: | Line 647: | ||
|align=center|6 | |align=center|6 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L110 |
|align=center| D124 | |align=center| D124 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 654: | Line 655: | ||
|align=center|6 | |align=center|6 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L111 |
|align=center| D104 | |align=center| D104 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 662: | Line 663: | ||
|align=center|9 | |align=center|9 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L112 |
|align=center| D104 | |align=center| D104 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 670: | Line 671: | ||
|align=center|9 | |align=center|9 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L113 |
|align=center| D126 | |align=center| D126 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 678: | Line 679: | ||
|align=center|9.5 | |align=center|9.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L114 |
|align=center| D126 | |align=center| D126 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 686: | Line 687: | ||
|align=center|9.5 | |align=center|9.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L115 |
|align=center| D105 | |align=center| D105 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 694: | Line 695: | ||
|align=center|- | |align=center|- | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L116 |
|align=center| D105 | |align=center| D105 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 702: | Line 703: | ||
|align=center|- | |align=center|- | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L117 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D117 | |align=center| D117 | ||
| Line 710: | Line 711: | ||
|align=center|10 | |align=center|10 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L118 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D121 | |align=center| D121 | ||
| Line 718: | Line 719: | ||
|align=center|11 | |align=center|11 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L119 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D113 | |align=center| D113 | ||
| Line 726: | Line 727: | ||
|align=center|11 | |align=center|11 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L120 |
|align=center| D106 | |align=center| D106 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 734: | Line 735: | ||
|align=center|3.5 | |align=center|3.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L121 |
|align=center| D106 | |align=center| D106 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 742: | Line 743: | ||
|align=center|3.5 | |align=center|3.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L122 |
|align=center| D107 | |align=center| D107 | ||
|align=center| D130 | |align=center| D130 | ||
| Line 750: | Line 751: | ||
|align=center|6.5 | |align=center|6.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L123 |
|align=center| D107 | |align=center| D107 | ||
|align=center| D131 | |align=center| D131 | ||
| Line 758: | Line 759: | ||
|align=center|6.5 | |align=center|6.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L124 |
|align=center| D102 | |align=center| D102 | ||
|align=center| D122 | |align=center| D122 | ||
| Line 766: | Line 767: | ||
|align=center|4 | |align=center|4 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L125 |
|align=center| D102 | |align=center| D102 | ||
|align=center| D1118 | |align=center| D1118 | ||
| Line 774: | Line 775: | ||
|align=center|1 | |align=center|1 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L126 |
|align=center| D102 | |align=center| D102 | ||
|align=center| D114 | |align=center| D114 | ||
| Line 782: | Line 783: | ||
|align=center|7 | |align=center|7 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| L127 |
|align=center| D125 | |align=center| D125 | ||
|align=center| D119 | |align=center| D119 | ||
| Line 790: | Line 791: | ||
|align=center|8 | |align=center|8 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C1 |
|align=center| D110 | |align=center| D110 | ||
|align=center| - | |align=center| - | ||
| Line 798: | Line 799: | ||
|align=center|14 | |align=center|14 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C2 |
|align=center| D129 | |align=center| D129 | ||
|align=center| - | |align=center| - | ||
| Line 806: | Line 807: | ||
|align=center|12 | |align=center|12 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C3 |
|align=center| D123 | |align=center| D123 | ||
|align=center| - | |align=center| - | ||
| Line 814: | Line 815: | ||
|align=center|12 | |align=center|12 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C4 |
|align=center| D103 | |align=center| D103 | ||
|align=center| - | |align=center| - | ||
| Line 822: | Line 823: | ||
|align=center|9 | |align=center|9 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C5 |
|align=center| D124 | |align=center| D124 | ||
|align=center| - | |align=center| - | ||
| Line 830: | Line 831: | ||
|align=center|10 | |align=center|10 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C6 |
|align=center| D104 | |align=center| D104 | ||
|align=center| - | |align=center| - | ||
| Line 838: | Line 839: | ||
|align=center|14 | |align=center|14 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C7 |
|align=center| D126 | |align=center| D126 | ||
|align=center| - | |align=center| - | ||
| Line 846: | Line 847: | ||
|align=center|14.5 | |align=center|14.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C8 |
|align=center| D105 | |align=center| D105 | ||
|align=center| - | |align=center| - | ||
| Line 854: | Line 855: | ||
|align=center|6 | |align=center|6 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C9 |
|align=center| D125 | |align=center| D125 | ||
|align=center| - | |align=center| - | ||
| Line 862: | Line 863: | ||
|align=center|15 | |align=center|15 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C10 |
|align=center| D106 | |align=center| D106 | ||
|align=center| - | |align=center| - | ||
| Line 870: | Line 871: | ||
|align=center|9.5 | |align=center|9.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C11 |
|align=center| D107 | |align=center| D107 | ||
|align=center| - | |align=center| - | ||
| Line 878: | Line 879: | ||
|align=center|13.5 | |align=center|13.5 | ||
|- | |- | ||
| - | | | + | |style="background: #ccccff;"| C12 |
|align=center| D102 | |align=center| D102 | ||
|align=center| - | |align=center| - | ||
| Line 887: | Line 888: | ||
|} | |} | ||
| + | == Purification of the B0034 and B0030 BioBricks == | ||
| - | + | ''We performed PCR on the B0034 BioBrick (MP 100) and the B0030 BioBrick (MP 120) to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.'' | |
| - | === Protocol === | + | === PCR Protocol === |
| - | For each samples, | + | ''For each samples,'' |
* 1 µl dNTP | * 1 µl dNTP | ||
| Line 900: | Line 902: | ||
* 1 µl Phusion | * 1 µl Phusion | ||
* 50 µl qsp H2O (33µl) | * 50 µl qsp H2O (33µl) | ||
| + | |||
| + | |||
| + | === Electrophoresis - Purification === | ||
| + | |||
| + | ''When the PCR cycles were finished,'' | ||
| + | |||
| + | * Add 10 µL of 6X loading dye. | ||
| + | * Load the samples (2 x 30 µL per sample) on a 1,5% agarose gel. | ||
| + | * Migration 30' at 100W. | ||
| + | |||
| + | |||
| + | ''After electrophoresis,'' | ||
| + | |||
| + | * Excision and purification of the bands corresponding to MP 100 and MP 120, using the QIAquick DNA Gel Extraction Kit (QIAGEN). | ||
| + | * Elution in 50 µL of water. | ||
| + | |||
| + | ''Because the intensity of the band corresponding to MP 120 was very low, we only continued with MP 100.'' | ||
| + | |||
| + | |||
| + | === DNA Digestion === | ||
| + | |||
| + | ''MP 100 was digested by EcoRI & SpeI (Forward Insert) or by XbaI & PstI (D100 : Backward Insert) | ||
| + | |||
| + | Digestion reaction (total volume : 50 µL)'' | ||
| + | |||
| + | * 25 µL DNA | ||
| + | * 5 µL buffer 2 (10X) | ||
| + | * 2 µL enzyme 1 | ||
| + | * 2 µL enzyme 2 | ||
| + | * 0.5 µL BSA (100X) | ||
| + | * 15,5 µL water | ||
| + | |||
| + | * The reaction was incubated 2 hours at 37°C. | ||
| + | * The samples (2 x 30 µL per sample) were then analysed by electrophoresis. | ||
| + | |||
| + | |||
| + | ===Electrophoresis=== | ||
| + | |||
| + | |||
| + | |||
| + | {| border="1" | ||
| + | |align=center|'''1''' | ||
| + | |align=center|'''2''' | ||
| + | |align=center|'''3''' | ||
| + | |align=center|'''4''' | ||
| + | |align=center|'''5''' | ||
| + | |align=center|'''6''' | ||
| + | |- | ||
| + | ! rowspan="1"|ladder | ||
| + | ! rowspan="1"|EcoRI & SpeI | ||
| + | ! rowspan="1"|EcoRI & SpeI | ||
| + | ! rowspan="1"| - | ||
| + | ! rowspan="1"|XbaI & PstI | ||
| + | ! rowspan="1"|XbaI & PstI | ||
| + | |} | ||
| + | |||
| + | [[Image:Image_gel.jpg|thumb|]] | ||
| + | |||
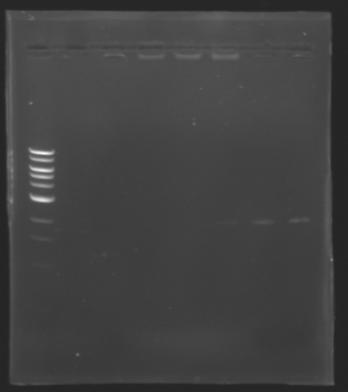
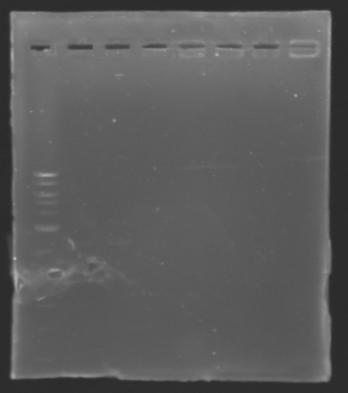
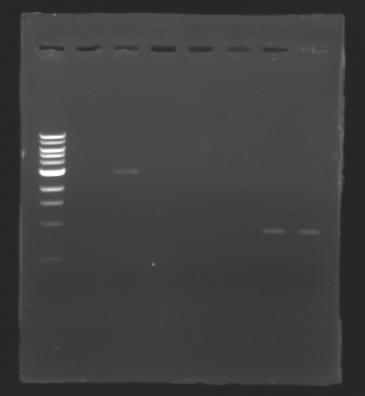
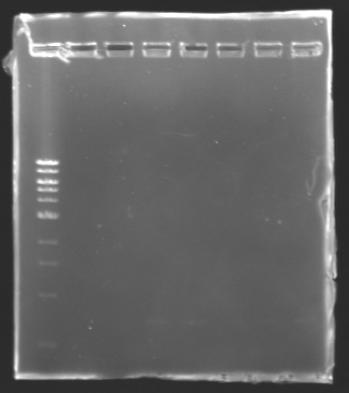
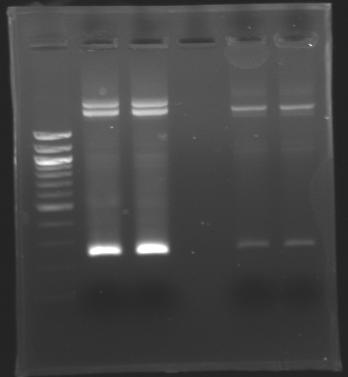
| + | The sequence of MP 100 (B0034) digested by EcoRI & SpeI (35 bp) or XbaI & PstI (34 bp) was too short and we didn't manage to visualise it on the gel. | ||
| + | |||
| + | ==> ''Conclusion'' : small parts like B0034 can't be cloned as an insert. | ||
Latest revision as of 16:59, 13 August 2008
|
DNA ExtractionResults of the extraction : Electrophoresisconditions of electrophoresis:
LigationProtocolFor each samples,
List of ligations
Purification of the B0034 and B0030 BioBricksWe performed PCR on the B0034 BioBrick (MP 100) and the B0030 BioBrick (MP 120) to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments. PCR ProtocolFor each samples,
Electrophoresis - PurificationWhen the PCR cycles were finished,
Because the intensity of the band corresponding to MP 120 was very low, we only continued with MP 100.
DNA DigestionMP 100 was digested by EcoRI & SpeI (Forward Insert) or by XbaI & PstI (D100 : Backward Insert) Digestion reaction (total volume : 50 µL)
Electrophoresis
The sequence of MP 100 (B0034) digested by EcoRI & SpeI (35 bp) or XbaI & PstI (34 bp) was too short and we didn't manage to visualise it on the gel. ==> Conclusion : small parts like B0034 can't be cloned as an insert. |
 "
"