Team:Paris/July 30
From 2008.igem.org
(→Results of transformations) |
(→Results of transformations) |
||
| (5 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Paris/Calendar_Links|July 29|July 31}} | {{Paris/Calendar_Links|July 29|July 31}} | ||
| - | |||
| Line 30: | Line 29: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L103 | |align="center"|L103 | ||
| - | |align="center"|Strong rbs - | + | |align="center"|Strong rbs - mRFP<br>D129 (BV) - D122 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L104 | |align="center"|L104 | ||
| Line 42: | Line 41: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L105 | |align="center"|L105 | ||
| Line 48: | Line 47: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L106 | |align="center"|L106 | ||
| Line 54: | Line 53: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L107 | |align="center"|L107 | ||
| - | |align="center"|Strongest promoter - ECFP<br>D103 (BV) - | + | |align="center"|Strongest promoter - ECFP<br>D103 (BV) - D130 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L108 n°2 <br>(the right one) | |align="center"|L108 n°2 <br>(the right one) | ||
| - | |align="center"|Strong promoter - gfp Tripart<br>D103 (BV) - | + | |align="center"|Strong promoter - gfp Tripart<br>D103 (BV) - D131 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L109 n°1 | |align="center"|L109 n°1 | ||
| Line 84: | Line 83: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L111 | |align="center"|L111 | ||
| Line 90: | Line 89: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L112 | |align="center"|L112 | ||
| - | |align="center"|Weak promoter - gfp<br>D104 (BV) - D131 (BI) | + | |align="center"|Weak promoter - gfp tripart<br>D104 (BV) - D131 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L113 | |align="center"|L113 | ||
| Line 114: | Line 113: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L116 | |align="center"|L116 | ||
| Line 120: | Line 119: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L117 | |align="center"|L117 | ||
| Line 126: | Line 125: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 30 | |align="center"|+/- 30 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L118 | |align="center"|L118 | ||
| Line 132: | Line 131: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 30 | |align="center"|+/- 30 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L119 | |align="center"|L119 | ||
| Line 138: | Line 137: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 30 | |align="center"|+/- 30 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L120 | |align="center"|L120 | ||
| - | |align="center"|tetR repressible - ECFP<br>D106 (BV) - D130 (BI) | + | |align="center"|tetR repressible promoter - ECFP<br>D106 (BV) - D130 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L121 | |align="center"|L121 | ||
| - | |align="center"| | + | |align="center"|tetR repressible promoter - gfp tripart<br>D106 (BV) - D131 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L122 | |align="center"|L122 | ||
| Line 159: | Line 158: | ||
|- | |- | ||
|align="center"|L123 | |align="center"|L123 | ||
| - | |align="center"|RBS lasI - | + | |align="center"|RBS lasI - gfp tripart<br>D107 (BV) - D131 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|1 | |align="center"|1 | ||
| Line 165: | Line 164: | ||
|- | |- | ||
|align="center"|L124 | |align="center"|L124 | ||
| - | |align="center"|Strongest RBS - | + | |align="center"|Strongest RBS - mRFP<br>D102 (BV) - D122 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L125 | |align="center"|L125 | ||
| Line 174: | Line 173: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|L126 | |align="center"|L126 | ||
| - | |align="center"|Strongest RBS (1)- LacR activator (+LVA)<br>D102 (BV) - | + | |align="center"|Strongest RBS (1)- LacR activator (+LVA)<br>D102 (BV) - D114 (BI) |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|no dish found!!! | |align="center"|no dish found!!! | ||
| Line 195: | Line 194: | ||
|- | |- | ||
|align="center"|C1 | |align="center"|C1 | ||
| - | |align="center"| | + | |align="center"|D110 |
| - | D110 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C2 | |align="center"|C2 | ||
| - | |align="center"| | + | |align="center"|D129 |
| - | D129 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|5 | |align="center"|5 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C3 | |align="center"|C3 | ||
| - | |align="center"| | + | |align="center"|D123 |
| - | D123 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C4 | |align="center"|C4 | ||
| - | |align="center"| | + | |align="center"|D103 |
| - | D103 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 20 | |align="center"|+/- 20 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C5 | |align="center"|C5 | ||
| - | |align="center"| | + | |align="center"|D124 |
| - | D124 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C6 | |align="center"|C6 | ||
| - | |align="center"| | + | |align="center"|D104 |
| - | D104 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C7 | |align="center"|C7 | ||
| - | |align="center"| | + | |align="center"|D126 |
| - | D126 | + | |
|align="center"|Kana | |align="center"|Kana | ||
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C8 | |align="center"|C8 | ||
| - | |align="center"| | + | |align="center"|D105 |
| - | D105 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 20 | |align="center"|+/- 20 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C9 | |align="center"|C9 | ||
| - | |align="center"| | + | |align="center"|D125 |
| - | D125 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C10 | |align="center"|C10 | ||
| - | |align="center"| | + | |align="center"|D106 |
| - | D106 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C11 | |align="center"|C11 | ||
| - | |align="center"| | + | |align="center"|D107 |
| - | D107 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|0 | |align="center"|0 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|C12 | |align="center"|C12 | ||
| - | |align="center"| | + | |align="center"|D102 |
| - | D102 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|+/- 10 | |align="center"|+/- 10 | ||
| - | |align="center"| | + | |align="center"|ok |
|- | |- | ||
|align="center"|Positive control | |align="center"|Positive control | ||
| - | |align="center"| | + | |align="center"|puc19 |
| - | puc19 | + | |
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|155 (transformation <br> efficiency:1.5*10^7/ug) | |align="center"|155 (transformation <br> efficiency:1.5*10^7/ug) | ||
| - | |align="center"| | + | |align="center"|ok |
|} | |} | ||
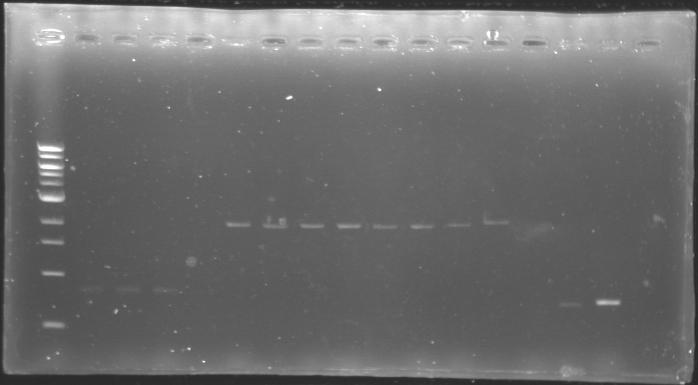
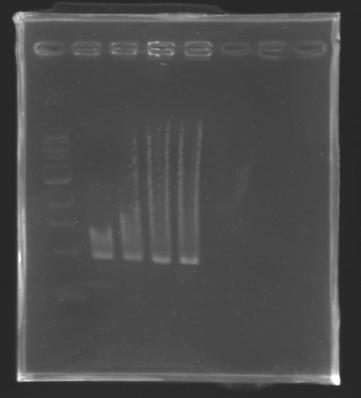
==Analysis of yesterday DNA digestion== | ==Analysis of yesterday DNA digestion== | ||
| - | The digested DNA of yesterday was analysed one more time by electrophoresis on a 0.8% agarose gel (about 30 minutes at 100 W). The ladder used was the 1 kb DNA ladder (New England Biolabs). 5 µL of each sample with 1 µL of loading dye were loaded. | + | ''The digested DNA of yesterday was analysed '''one more time''' by electrophoresis on a '''0.8% agarose gel''' (about 30 minutes at 100 W).'' |
| + | * The ladder used was the 1 kb DNA ladder (New England Biolabs). | ||
| + | * 5 µL of each sample with 1 µL of loading dye were loaded.' | ||
[[Image:KR000082.jpg|thumb|]] | [[Image:KR000082.jpg|thumb|]] | ||
| - | |||
| - | |||
{| border="1" | {| border="1" | ||
| Line 429: | Line 415: | ||
|} | |} | ||
| - | + | ==> '''Conclusion :'''Each of the samples was succesfully digested and purified except for the sample D108. It seems that the QIAprep columms (from the QIAGEN Minipreps kit) can be used instead of the QIAquick columms (for DNA Gel Extraction). | |
| - | Each of the samples was succesfully digested and purified except for the sample D108. It seems that the QIAprep columms (from the QIAGEN Minipreps kit) can be used instead of the QIAquick columms (for DNA Gel Extraction). | + | |
| - | + | ||
| Line 479: | Line 463: | ||
* 10µl of screening PCR (gel n°3(1), 13, 14) | * 10µl of screening PCR (gel n°3(1), 13, 14) | ||
* migration ~30min at 100W on '''0,8%''' gel | * migration ~30min at 100W on '''0,8%''' gel | ||
| + | |||
===Results=== | ===Results=== | ||
Latest revision as of 17:03, 13 August 2008
|
Results of transformations
Analysis of yesterday DNA digestionThe digested DNA of yesterday was analysed one more time by electrophoresis on a 0.8% agarose gel (about 30 minutes at 100 W).
==> Conclusion :Each of the samples was succesfully digested and purified except for the sample D108. It seems that the QIAprep columms (from the QIAGEN Minipreps kit) can be used instead of the QIAquick columms (for DNA Gel Extraction).
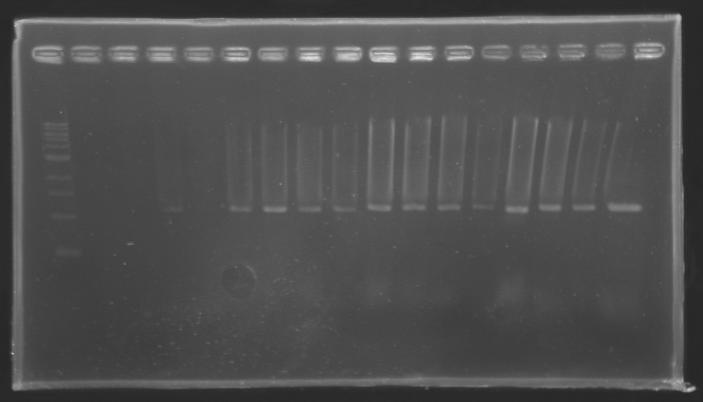
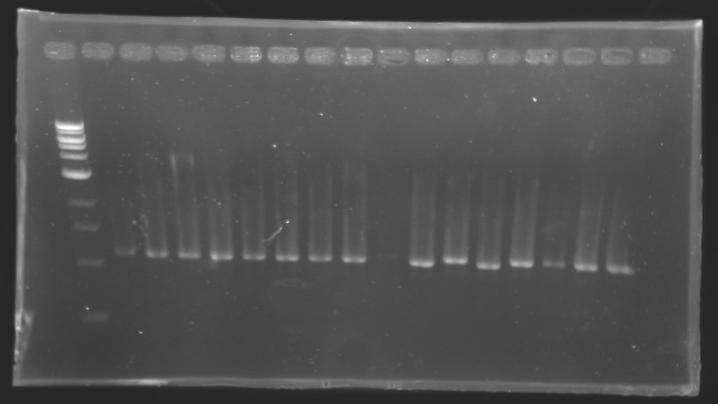
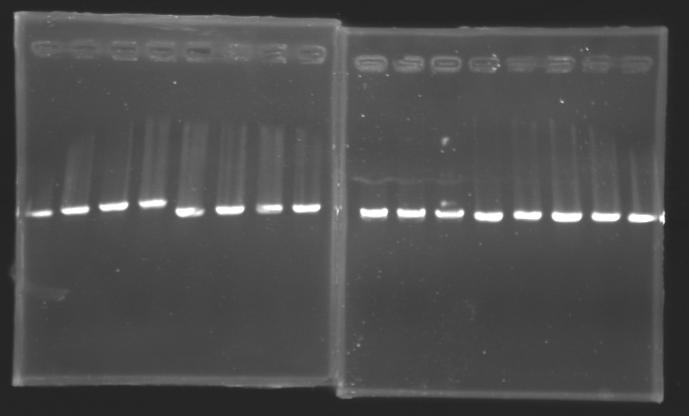
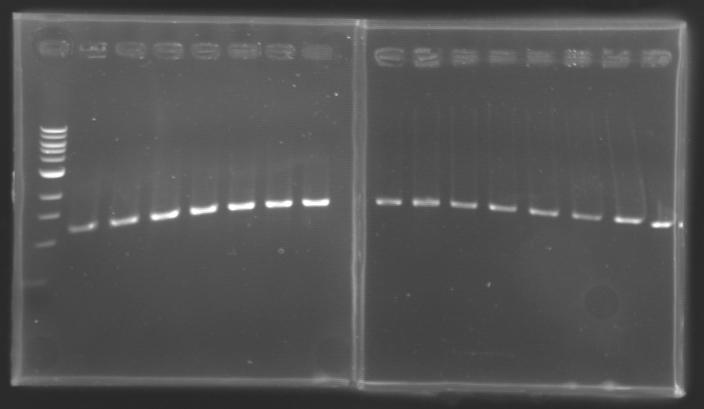
PCR Screening of Ligation TransformantsUse of 8 clones of Ligation transformants for screening PCR
Protocol of screening PCR
Conditions of electrophoresis
Results
But we don't observe results for L102(3), L102(6), L103(4), L106(1), L106(2), L106(4), L111(1) Migration of an another gel for this sample...
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"