Team:Paris/August 12
From 2008.igem.org
(→Glycerol Stocks) |
(→Results of the transformations we did yesterday) |
||
| (52 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Paris/Calendar_Links|August 11|August 13}} | {{Paris/Calendar_Links|August 11|August 13}} | ||
| - | |||
| - | + | =='''Digestion and ligation of the PCR we did yesterday'''== | |
| + | Yesterday we amplified ''flhD'', ''flhC'', ''flhDC'' with its promoter and ''E040 RBS+'' | ||
| + | Before the digestion, we have to determine the DNA concentration of the templates. | ||
| - | + | ==='''Measurement of DNA concentration'''=== | |
| - | {| | + | We used the biophotometer. We put 5 µL of template DNA in 95 µL of water. The Blank was made with 5 µL of EB Buffer (the one that was used for the elution of the DNA) in 95 µL of water. |
| - | | | + | {| |- style="text-align: center;" Border="2" |
| - | | | + | |- style="text-align: center;" |
| - | |align="center"|'' | + | |'''Name''' |
| - | | | + | |'''Template''' |
| - | | | + | |'''Concentration '''<br>(µg/µL) |
| - | |align="center"| | + | |'''Ratio DO260/280''' |
| - | | | + | |- style="text-align: center;" |
| + | |PCR 130 | ||
| + | |E0240 RBS + | ||
| + | |0.44 | ||
| + | |nd | ||
| + | |- style="text-align: center;" | ||
| + | |PCR 131 | ||
| + | |''flhD'' RBS - | ||
| + | |0.06 | ||
| + | |nd | ||
| + | |- style="text-align: center;" | ||
| + | |align="center"| PCR 132 | ||
| + | | ''flhC'' RBS - | ||
| + | |align="center"| 0.12 | ||
| + | |nd | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"| PCR 133 |
| - | |align="center"| | + | |''flhDC'' with promoter |
| + | |align="center"| 0.08 | ||
| + | |nd | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"| MP 142 |
| - | |align="center"| | + | | pSB3K3 |
| + | |align="center"| 0.04 | ||
| + | |nd | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"| MP 122 |
| - | |align="center"| | + | |pSB1A2 |
| + | |align="center"| 0.2 | ||
| + | |nd | ||
| + | |} | ||
| + | |||
| + | ==='''Digestion'''=== | ||
| + | ====Protocol==== | ||
| + | {| Border="2" | ||
| + | |align="center"|'''Digestion name''' | ||
| + | |align="center"|'''Template DNA''' | ||
| + | |align="center"|''' Enzymes ''' | ||
| + | |align="center"|'''Volume of DNA''' | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"|D 140 |
| - | |align="center"| | + | |align="center"|PCR 130 - E0240 RBS + |
| - | | | + | |align="center"|PstI |
| + | |align="center"|5 µL | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"|D 141 |
| - | |align="center"| | + | |align="center"|PCR 131 - flhD rbs - |
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"|D 142 |
| - | |align="center"| | + | |align="center"|PCR 132 - flhC rbs - |
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"|D 143 |
| - | |align="center"| | + | |align="center"|PCR 133 - flhDC + prom |
| - | + | |align="center"|EcoRI-SpeI | |
| - | |align="center"| | + | |align="center"|5 µL |
| - | |align="center"| | + | |
| - | + | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"|D 144 |
| - | |align="center"| | + | |align="center"|MP 142 - pSB3K3 |
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"|D 145 |
| - | |align="center"| | + | |align="center"|MP122 - pSB1A2 |
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
| + | |} | ||
| + | |||
| + | * X µL of Template DNA | ||
| + | * Buffer (n°2) 10X : 3µL | ||
| + | * BSA 100X : 0.3µL | ||
| + | * Pure water qsp 30 µL | ||
| + | * 1 µL of each enzyme | ||
| + | |||
| + | * Incubate during about 3h at 37°C, then 20 minutes at 65°C (to inactivate the enzymes). | ||
| + | * Exclusively for D 144 : 2 µL of Antarctic Phosphatase was added, 30 min at 37°C then 5 min at 65°C. | ||
| + | |||
| + | ====Results of the digestion==== | ||
| + | |||
| + | For the digestion after PCR, an electrophoresis is useless : we can not separate DNA fragments that have | ||
| + | 10 bp of difference.<br> | ||
| + | Exclusively for D 145 a gel extraction was made because there was two fragments, the standard [[Team:Paris/Notebook/Protocols#Extraction|protocol]] | ||
| + | was used (the picture is missing because we hadn't the USB key). | ||
| + | The DNA purification after gel extraction was done according the [[Team:Paris/Notebook/Protocols#Purification_.28Kit_Promega.29|standard protocol]]. | ||
| + | |||
| + | ==='''Ligation'''=== | ||
| + | {| Border="2" | ||
| + | |align="center"|'''Ligation name''' | ||
| + | |align="center"|'''Insert name ''' | ||
| + | |align="center"|'''Volume of insert µL''' | ||
| + | |align="center"|'''Vector name''' | ||
| + | |align="center"|'''Volume of Vector µL''' | ||
|- | |- | ||
| - | |align="center"| | + | |align="center"|L 139 |
| - | |align="center"| | + | |align="center"|D 140 (E0240 RBS +) |
| + | |align="center"| 3 | ||
| + | |align="center"|D 144 (pSB3K3) | ||
| + | |align="center"| 3 | ||
| + | |- | ||
| + | |align="center"|L 140 | ||
| + | |align="center"|D 141 (flhD) | ||
| + | |align="center"| 3 | ||
| + | |align="center"|D 145 (pSB1A2) | ||
| + | |align="center"| 3 | ||
| + | |- | ||
| + | |align="center"|L 141 | ||
| + | |align="center"|D 142 (flhC) | ||
| + | |align="center"| 3 | ||
| + | |align="center"|D 145 (pSB1A2) | ||
| + | |align="center"| 3 | ||
| + | |- | ||
| + | |align="center"|L 142 | ||
| + | |align="center"|D 143 (flhDC + promoter) | ||
| + | |align="center"| 3 | ||
| + | |align="center"|D 145 (pSB1A2) | ||
| + | |align="center"| 3 | ||
|} | |} | ||
| + | |||
| + | |||
| + | REMARKS : <br> | ||
| + | [https://2008.igem.org/Image:Cyprien-Maisonnier.jpg Someone] forgot to do the autoligation control, which is a key step in the evaluation of the ligation process. | ||
| + | |||
| + | ==''' Cloning on fliL promoter''' == | ||
| + | ===Protocol=== | ||
| + | We used the Taq polymerase to amplify this promoter. | ||
| + | |||
| + | * '''Preparation of the template''' : | ||
| + | Resuspension of 1 colony ''E.coli'' K12 strain MG 1655 in 100µl of water. | ||
| + | |||
| + | * '''Preparation of PCR mix''' : | ||
| + | 1 µl O 124 | ||
| + | <br> 1 µl O 125 | ||
| + | <br> 1 µl template DNA | ||
| + | <br> 25 µL Quick Load Taq polymerase Mix | ||
| + | <br> 22 µL pure water | ||
| + | ===Result=== | ||
| + | We did an electrophoresis to check if our amplicon has the right size.<br> | ||
| + | Actually, the electrophoresis did not show anything, not even the DNA ladder.<br> | ||
| + | We decided to do again the electrophoresis tomorrow morning.<br> | ||
== Minipreps: Plasmid extraction== | == Minipreps: Plasmid extraction== | ||
| - | * [https://2008.igem.org/Team:Paris/Notebook/Protocols#Minipreps_.28Kit_Qiagen.29 Protocol] (see #'''3''') Experiments done by QIAcube | + | * [https://2008.igem.org/Team:Paris/Notebook/Protocols#Minipreps_.28Kit_Qiagen.29 Protocol] (see # '''3''') Experiments done by QIAcube |
* '''List of Minipreps''' | * '''List of Minipreps''' | ||
| Line 61: | Line 167: | ||
|'''Name''' | |'''Name''' | ||
|'''Ligation''' | |'''Ligation''' | ||
| - | |||
|'''Description''' | |'''Description''' | ||
| + | |'''Biobricks''' | ||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | |MP144 | + | |MP144.1 |
|L128.1 | |L128.1 | ||
| - | |pFlgA-RFP<br>D132 (FI) - D136 (FV) | + | | rowspan="4"| pFlgA-RFP<br>D132 (FI) - D136 (FV) |
| - | |[[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] | + | | rowspan="4"| [[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] |
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP144.2 |
|L128.2 | |L128.2 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP144.3 |
|L128.3 | |L128.3 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP144.4 |
|L128.4 | |L128.4 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP145.1 |
|L129.1 | |L129.1 | ||
| - | |pFlgB-RFP<br>D133 (FI) - D136 (FV) | + | | rowspan ="4"| pFlgB-RFP<br>D133 (FI) - D136 (FV) |
| - | |[[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] | + | | rowspan ="4"| [[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] |
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP145.2 |
|L129.2 | |L129.2 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP145.6 |
|L129.6 | |L129.6 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP145.7 |
|L129.7 | |L129.7 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP146.1 |
|L130.1 | |L130.1 | ||
| - | |pFlhB-RFP<br>D134 (FI) - D136 (FV) | + | | rowspan="4"| pFlhB-RFP<br>D134 (FI) - D136 (FV) |
| - | |[[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] | + | | rowspan="4"| [[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] |
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP146.2 |
|L130.2 | |L130.2 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP146.7 |
|L130.7 | |L130.7 | ||
| - | |||
| - | |||
|- style="text-align: center;" | |- style="text-align: center;" | ||
| - | | | + | |MP146.8 |
|L130.8 | |L130.8 | ||
| - | |||
| - | |||
|} | |} | ||
| + | |||
| + | ==Glycerol Stocks== | ||
| + | |||
| + | * [https://2008.igem.org/Team:Paris/Notebook/Protocols#Glycerol_Stocks Protocol] (see #'''2''') | ||
| + | |||
| + | * '''List of Stocks''' | ||
| + | {||- style="text-align: center;" border="1" | ||
| + | |align="center"|'''Strain''' | ||
| + | |align="center"|'''Ligation''' | ||
| + | |align="center"|'''Description''' | ||
| + | |align="center"|'''Biobricks''' | ||
| + | |- | ||
| + | |align="center"|S143.1 | ||
| + | |align="center"|L128.1 | ||
| + | |rowspan="4" |pFlgA-RFP<br>D132 (FI) - D136 (FV) | ||
| + | |rowspan="4" |[[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] | ||
| + | |- | ||
| + | |align="center"|S143.2 | ||
| + | |align="center"|L128.2 | ||
| + | |- | ||
| + | |align="center"|S143.3 | ||
| + | |align="center"|L128.3 | ||
| + | |- | ||
| + | |align="center"|S143.4 | ||
| + | |align="center"|L128.4 | ||
| + | |- | ||
| + | |align="center"|S144.1 | ||
| + | |align="center"|L129.1 | ||
| + | |rowspan="4" |pFlgB-RFP<br>D133 (FI) - D136 (FV) | ||
| + | |rowspan="4" |[[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] | ||
| + | |- | ||
| + | |align="center"|S144.2 | ||
| + | |align="center"|L129.2 | ||
| + | |- | ||
| + | |align="center"|S144.6 | ||
| + | |align="center"|L129.6 | ||
| + | |- | ||
| + | |align="center"|S144.7 | ||
| + | |align="center"|L129.7 | ||
| + | |- | ||
| + | |align="center"|S145.1 | ||
| + | |align="center"|L130.1 | ||
| + | |rowspan="4" |pFlhB-RFP<br>D134 (FI) - D136 (FV) | ||
| + | |rowspan="4" |[[Image:Part_icon_regulatory.png]][[Image:Part_icon_reporter.png]] | ||
| + | |- | ||
| + | |align="center"|S145.2 | ||
| + | |align="center"|L130.2 | ||
| + | |- | ||
| + | |align="center"|S145.7 | ||
| + | |align="center"|L130.7 | ||
| + | |- | ||
| + | |align="center"|S145.8 | ||
| + | |align="center"|L130.8 | ||
| + | |} | ||
| + | |||
| + | =='''Results of the transformations we did [[Team:Paris/August_11|yesterday]]'''== | ||
| + | |||
| + | {| Border="2" | ||
| + | |align="center"|'''Ligation name''' | ||
| + | |align="center"|''' What's in it ? ''' | ||
| + | |align="center"|'''Number of UFC''' | ||
| + | |- | ||
| + | |align="center"|L 132 | ||
| + | |align="center"|flhDC (gene) | ||
| + | |align="center"|6 | ||
| + | |- | ||
| + | |align="center"|L 133 | ||
| + | |align="center"|OmpR* | ||
| + | |align="center"|1 | ||
| + | |- | ||
| + | |align="center"|L 134 | ||
| + | |align="center"|EnvZ* | ||
| + | |align="center"|11 | ||
| + | |- | ||
| + | |align="center"|L 135 | ||
| + | |align="center"|pflgA | ||
| + | |align="center"|20 | ||
| + | |- | ||
| + | |align="center"|L 136 | ||
| + | |align="center"|pflgB | ||
| + | |align="center"|100 | ||
| + | |- | ||
| + | |align="center"|L 137 | ||
| + | |align="center"|pflhB | ||
| + | |align="center"|100 | ||
| + | |- | ||
| + | |align="center"|L 138 | ||
| + | |align="center"|E0240 | ||
| + | |align="center"|1 | ||
| + | |} | ||
| + | |||
| + | =='''PCR screening of the transformations we did [[Team:Paris/August_11|yesterday]]'''== | ||
| + | |||
| + | ==Concentration of the MiniPreps== | ||
| + | |||
| + | {|border="1" | ||
| + | |align="center"|'''Miniprep''' | ||
| + | |align="center"|'''Concentration (µg/mL)''' | ||
| + | |align="center"|'''Ratio 260/280''' | ||
| + | |- | ||
| + | |align="center"|MP144.1 | ||
| + | |align="center"|127 | ||
| + | |align="center"|1.67 | ||
| + | |- | ||
| + | |align="center"|MP144.2 | ||
| + | |align="center"|166 | ||
| + | |align="center"|1.66 | ||
| + | |- | ||
| + | |align="center"|MP144.3 | ||
| + | |align="center"|163 | ||
| + | |align="center"|1.57 | ||
| + | |- | ||
| + | |align="center"|MP144.4 | ||
| + | |align="center"|154 | ||
| + | |align="center"|1.63 | ||
| + | |- | ||
| + | |align="center"|MP145.1 | ||
| + | |align="center"|136 | ||
| + | |align="center"|1.67 | ||
| + | |- | ||
| + | |align="center"|MP145.2 | ||
| + | |align="center"|151 | ||
| + | |align="center"|1.63 | ||
| + | |- | ||
| + | |align="center"|MP145.6 | ||
| + | |align="center"|163 | ||
| + | |align="center"|1.67 | ||
| + | |- | ||
| + | |align="center"|MP145.7 | ||
| + | |align="center"|188 | ||
| + | |align="center"|1.57 | ||
| + | |- | ||
| + | |align="center"|MP146.1 | ||
| + | |align="center"|298 | ||
| + | |align="center"|1.70 | ||
| + | |- | ||
| + | |align="center"|MP146.2 | ||
| + | |align="center"|142 | ||
| + | |align="center"|1.64 | ||
| + | |- | ||
| + | |align="center"|MP146.7 | ||
| + | |align="center"|170 | ||
| + | |align="center"|1.52 | ||
| + | |- | ||
| + | |align="center"|MP146.8 | ||
| + | |align="center"|143 | ||
| + | |align="center"|1.55 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | ==New PCR screening with the right primers== | ||
| + | |||
| + | Transformants with pFlgA, pFlgB and pFlhB cloned into J61002 are analysed by PCR but this time with the right primers: '''VF2''' (O18) and '''VR''' (O19). | ||
| + | *template: bacteria from glycerol stock | ||
| + | *positive control: J61002 (with ptet-mRFP) | ||
| + | *negative control: no template | ||
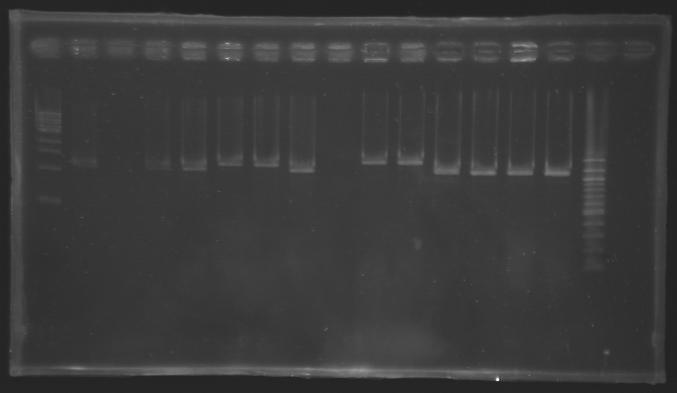
| + | ===Electrophoresis=== | ||
| + | *1% agarose gel | ||
| + | *10 µL of each sample loaded | ||
| + | |||
| + | {|border="1" | ||
| + | |align="center"|'''well n°''' | ||
| + | |align="center"|1 | ||
| + | |align="center"|2 | ||
| + | |align="center"|3 | ||
| + | |align="center"|4 | ||
| + | |align="center"|5 | ||
| + | |align="center"|6 | ||
| + | |align="center"|7 | ||
| + | |align="center"|8 | ||
| + | |align="center"|9 | ||
| + | |align="center"|10 | ||
| + | |align="center"|11 | ||
| + | |align="center"|12 | ||
| + | |align="center"|13 | ||
| + | |align="center"|14 | ||
| + | |align="center"|15 | ||
| + | |align="center"|16 | ||
| + | |- | ||
| + | |align="center"|'''sample''' | ||
| + | |align="center"|1 kb DNA ladder | ||
| + | |align="center"|positive control | ||
| + | |align="center"|negative control | ||
| + | |colspan="4"|<center>L128 ('''pFlgA''')</center> | ||
| + | |colspan="4"|<center>L129 ('''pFlgB''')</center> | ||
| + | |colspan="4"|<center>L130 ('''pFlhB''')</center> | ||
| + | |align="center"|100 bp DNA ladder | ||
| + | |- | ||
| + | |align="center"|'''clone n°''' | ||
| + | |colspan="3"| | ||
| + | |align="center"|1 | ||
| + | |align="center"|2 | ||
| + | |align="center"|3 | ||
| + | |align="center"|4 | ||
| + | |align="center"|1 | ||
| + | |align="center"|2 | ||
| + | |align="center"|6 | ||
| + | |align="center"|7 | ||
| + | |align="center"|1 | ||
| + | |align="center"|2 | ||
| + | |align="center"|7 | ||
| + | |align="center"|8 | ||
| + | |align="center"| | ||
| + | |- | ||
| + | |align="center"|'''red fluorescence''' | ||
| + | |colspan="3"| | ||
| + | |style="background: #ff6d73"|<center>yes</center> | ||
| + | |style="background: #ff6d73"|<center>yes</center> | ||
| + | |align="center"|no | ||
| + | |align="center"|no | ||
| + | |style="background: #ff6d73"|<center>yes</center> | ||
| + | |style="background: #ff6d73"|<center>yes</center> | ||
| + | |align="center"|no | ||
| + | |align="center"|no | ||
| + | |style="background: #ff6d73"|<center>yes</center> | ||
| + | |align="center"|no | ||
| + | |align="center"|no | ||
| + | |align="center"|no | ||
| + | |align="center"| | ||
| + | |- | ||
| + | |align="center"|'''expected size''' | ||
| + | |align="center"| | ||
| + | |align="center"|'''1161 bp''' | ||
| + | |align="center"|0 bp | ||
| + | |colspan="4"|<center>'''1339 bp'''</center> | ||
| + | |colspan="4"|<center>'''1339 bp'''</center> | ||
| + | |colspan="4"|<center>'''1338 bp'''</center> | ||
| + | |align="center"| | ||
| + | |- | ||
| + | |align="center"|'''measured size''' | ||
| + | |align="center"| | ||
| + | |align="center"|1,2 kb | ||
| + | |align="center"|0 kb | ||
| + | |align="center"|1,2 kb | ||
| + | |align="center"|1,2 kb | ||
| + | |style="background: #cbff7B"|<center>1,3 kb</center> | ||
| + | |style="background: #cbff7B"|<center>1,3 kb</center> | ||
| + | |align="center"|1,2 kb | ||
| + | |align="center"|0 kb | ||
| + | |style="background: #cbff7B"|<center>1,4 kb</center> | ||
| + | |style="background: #cbff7B"|<center>1,4 kb</center> | ||
| + | |align="center"|1,2 kb | ||
| + | |align="center"|1,2 kb | ||
| + | |align="center"|1,2 kb | ||
| + | |align="center"|1,2 kb | ||
| + | |align="center"| | ||
| + | |} | ||
| + | [[Image:Screening-PCR-080812.JPG|thumb]] | ||
Latest revision as of 11:00, 15 August 2008
|
Digestion and ligation of the PCR we did yesterdayYesterday we amplified flhD, flhC, flhDC with its promoter and E040 RBS+ Before the digestion, we have to determine the DNA concentration of the templates. Measurement of DNA concentrationWe used the biophotometer. We put 5 µL of template DNA in 95 µL of water. The Blank was made with 5 µL of EB Buffer (the one that was used for the elution of the DNA) in 95 µL of water.
DigestionProtocol
Results of the digestion For the digestion after PCR, an electrophoresis is useless : we can not separate DNA fragments that have
10 bp of difference.
Ligation
REMARKS : Cloning on fliL promoterProtocolWe used the Taq polymerase to amplify this promoter.
Resuspension of 1 colony E.coli K12 strain MG 1655 in 100µl of water.
1 µl O 124
ResultWe did an electrophoresis to check if our amplicon has the right size. Minipreps: Plasmid extraction
Glycerol Stocks
Results of the transformations we did yesterday
PCR screening of the transformations we did yesterdayConcentration of the MiniPreps
New PCR screening with the right primersTransformants with pFlgA, pFlgB and pFlhB cloned into J61002 are analysed by PCR but this time with the right primers: VF2 (O18) and VR (O19).
Electrophoresis
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"