Team:Paris/August 16
From 2008.igem.org
(Difference between revisions)
(→Digestion check from yesterday) |
|||
| (34 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Paris/Calendar_Links|August 15|August 17}} | {{Paris/Calendar_Links|August 15|August 17}} | ||
| - | ==''' Analysis of the transformation we did [[Team:Paris/August_15#Transformation_of_the_ligations_we_did__yesterday| yesterday]] | + | =Construction of OmpR*+RBS and EnvZ*+RBS: '''Ligations= |
| + | ==='''Cleaning of the DNA after the digestion'''=== | ||
| + | We used the QIAcube to wash the DNA, following the [[Team:Paris/Notebook/Protocols#Purification_.28Kit_Promega.29|standard protocol.]] | ||
| + | |||
| + | ==='''Measure of DNA concentration of the digestion products'''=== | ||
| + | We used the biophotometer.<br> | ||
| + | '''Settings:''' | ||
| + | *10 µL of template DNA in 50 µL of pure water | ||
| + | *Blank : 10 µL of EB buffer in 50 µL of water. | ||
| + | {||- style="text-align: center;" border="1" | ||
| + | |align="center"|'''Digestion name''' | ||
| + | |align="center"|'''What's in ?''' | ||
| + | |'''Enzymes''' | ||
| + | |align="center"|'''DNA C° (ng/µL)''' | ||
| + | |- | ||
| + | |align="center"|D 158 | ||
| + | |align="center"| OmpR* | ||
| + | |XbaI-PstI | ||
| + | |align="center"| 12 | ||
| + | |- | ||
| + | |align="center"|D 159 | ||
| + | |align="center"| EnvZ* | ||
| + | |XbaI-PstI | ||
| + | |align="center"| 7 | ||
| + | |- | ||
| + | |align="center"|D 102 | ||
| + | |align="center"|B0034 | ||
| + | |SpeI-PstI | ||
| + | |align="center"| 16 | ||
| + | |} | ||
| + | |||
| + | ==='''List of ligations'''=== | ||
| + | |||
| + | * We ligated the DNA following the [[Team:Paris/Notebook/Protocols#Ligation|standard protocol]]. | ||
| + | * T5 is the autoligation control for L148 and L149. | ||
| + | |||
| + | {| style="text-align: center;" Border="2" | ||
| + | |'''Ligation name''' | ||
| + | |'''Insert name ''' | ||
| + | |'''V<sub>insert</sub> µL''' | ||
| + | |'''Vector name''' | ||
| + | |'''V<sub>Vector</sub> µL''' | ||
| + | |- | ||
| + | |L 148 (Amp) | ||
| + | |D 158 (OmpR*) | ||
| + | |4 | ||
| + | |D 102 (B0034) | ||
| + | |3 | ||
| + | |- | ||
| + | |L 149 (Amp) | ||
| + | |D 159 (EnvZ) | ||
| + | |7 | ||
| + | |D 102 (B0034) | ||
| + | |3 | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | =Creation of a registry of pFliL, pFlhDC, and ''FlhDC''= | ||
| + | == Analysis of the transformation we did [[Team:Paris/August_15#Transformation_of_the_ligations_we_did__yesterday| yesterday]]== | ||
L143, L144, T1 and T2 showed no colonies. The positive control with pUC19 worked well. | L143, L144, T1 and T2 showed no colonies. The positive control with pUC19 worked well. | ||
<br>We suppose that the ligation did not work. We will do it again today. | <br>We suppose that the ligation did not work. We will do it again today. | ||
| - | == | + | ==Cleaning of the DNA after the digestion== |
| + | We used the QIAcube to wash the DNA, following the [[Team:Paris/Notebook/Protocols#Purification_.28Kit_Promega.29|standard protocol.]] | ||
| - | ==''' | + | ==Measure of DNA concentration of the digestion products== |
| + | We used the biophotometer.<br> | ||
| + | '''Settings:''' | ||
| + | *10 µL of template DNA in 50 µL of pure water | ||
| + | *Blank : 10 µL of EB buffer in 50 µL of water. | ||
| + | {| Border="2" | ||
| + | |align="center"|'''Digestion name''' | ||
| + | |align="center"|'''What's in ?''' | ||
| + | |align="center"|'''DNA concentration (ng/µL)''' | ||
| + | |- | ||
| + | |align="center"|D 149 | ||
| + | |align="center"|pfliL | ||
| + | |align="center"| 2 | ||
| + | |- | ||
| + | |align="center"|D 150 | ||
| + | |align="center"|pfliL | ||
| + | |align="center"| 3 | ||
| + | |- | ||
| + | |align="center"|D 151 | ||
| + | |align="center"|pSB3K3 | ||
| + | |align="center"| 12 | ||
| + | |- | ||
| + | |align="center"|D 152 | ||
| + | |align="center"|pSB3K3 | ||
| + | |align="center"| 20 | ||
| + | |- | ||
| + | |align="center"|D 153 | ||
| + | |align="center"|gene flhDC | ||
| + | |align="center"| 7 | ||
| + | |- | ||
| + | |align="center"|D 154 | ||
| + | |align="center"|gene flhDC | ||
| + | |align="center"| 12 | ||
| + | |- | ||
| + | |align="center"|D 155 | ||
| + | |align="center"|pflhDC | ||
| + | |align="center"| 16 | ||
| + | |- | ||
| + | |align="center"|D 136 | ||
| + | |align="center"|j61002 | ||
| + | |align="center"| 12 | ||
| + | |- | ||
| + | |align="center"|D 145 | ||
| + | |align="center"|pSB1A2 | ||
| + | |align="center"| 21 | ||
| + | |} | ||
| + | |||
| + | ==List of ligations== | ||
| + | |||
| + | * We ligated the DNA following the [[Team:Paris/Notebook/Protocols#Ligation|standard protocol]]. | ||
| + | * T1, T2, T3 and T4 are the autoligation controls for L 143, L144, L145 and L147 | ||
| + | |||
| + | {| style="text-align: center;" Border="2" | ||
| + | |'''Ligation name''' | ||
| + | |'''Insert name ''' | ||
| + | |'''V<sub>insert</sub> µL''' | ||
| + | |'''Vector name''' | ||
| + | |'''V<sub>Vector</sub> µL''' | ||
| + | |- | ||
| + | |L 143 (Kan) | ||
| + | |D 149 (pfliL) | ||
| + | |8 | ||
| + | |D 137 (pSB3K3) | ||
| + | |4 | ||
| + | |- | ||
| + | |L 144 (Kan) | ||
| + | |D 150 (pfliL) | ||
| + | |5 | ||
| + | |D 152 (pSB3K3) | ||
| + | |2.5 | ||
| + | |- | ||
| + | |L 145 (Amp) | ||
| + | |D 153 (g flhDC) | ||
| + | |10 | ||
| + | |D 145 (pSB1A2) | ||
| + | |2.5 | ||
| + | |- | ||
| + | |L 146 (Amp) | ||
| + | |D 154 (g flhDC) | ||
| + | |5 | ||
| + | |D 145 (pSB1A2) | ||
| + | |2.5 | ||
| + | |- | ||
| + | |L 147 (Amp) | ||
| + | |D 155 (p flhDC) | ||
| + | |1 | ||
| + | |D 136 (J61002) | ||
| + | |4 | ||
| + | |} | ||
| + | |||
| + | =Construction of pLas-TetR-GFP tripart & rbs-LasR-dble ter= | ||
| + | ==Transformation== | ||
[[Team:Paris/Notebook/Protocols#Transformation |Transformation protocol]] | [[Team:Paris/Notebook/Protocols#Transformation |Transformation protocol]] | ||
| Line 39: | Line 190: | ||
|pUC19 | |pUC19 | ||
|Amp | |Amp | ||
| + | |} | ||
| + | |||
| + | |||
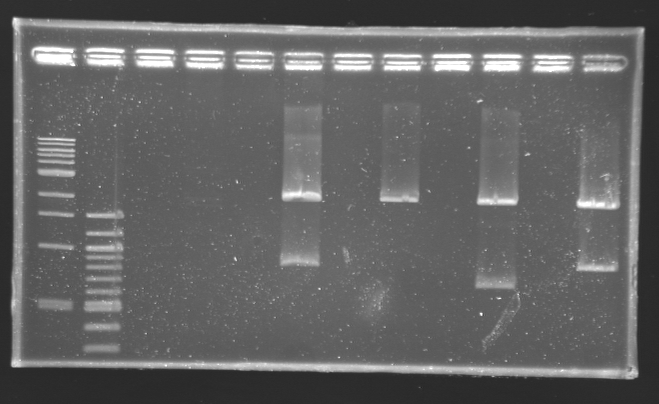
| + | ==Digestion check from yesterday== | ||
| + | [[Image:16-08-08.png|thumb|]] | ||
| + | [[Team:Paris/Notebook/Protocols#Digestion|Protocol Digestion]] | ||
| + | |||
| + | {| border="1" style="text-align: center" | ||
| + | |Digestion name | ||
| + | |Plasmid | ||
| + | |Description | ||
| + | |Miniprep used | ||
| + | |Expected size | ||
| + | |Measured size | ||
| + | |- | ||
| + | | | ||
| + | |MP3 | ||
| + | |B0015 (double terminator B0010-B0012) - FV | ||
| + | |4 | ||
| + | |No bands | ||
| + | |- | ||
| + | |D164 | ||
| + | |MP101 | ||
| + | |promoter J23101 - BV | ||
| + | |1 | ||
| + | | | ||
| + | |style="background: #cbff7B"| | ||
| + | |- | ||
| + | |D161 | ||
| + | |MP104 | ||
| + | |PTet (Tet promoter) - FI | ||
| + | |1 | ||
| + | | | ||
| + | |style="background: #cbff7B"| | ||
| + | |- | ||
| + | |D162 | ||
| + | |MP114 | ||
| + | |TetR - BI | ||
| + | |1 | ||
| + | | | ||
| + | |style="background: #cbff7B"| | ||
| + | |- | ||
| + | |D163 | ||
| + | |MP143 | ||
| + | |gfp generator - BV | ||
| + | |2 | ||
| + | | | ||
| + | |style="background: #cbff7B"| | ||
|} | |} | ||
Latest revision as of 18:08, 4 September 2008
Construction of OmpR*+RBS and EnvZ*+RBS: LigationsCleaning of the DNA after the digestionWe used the QIAcube to wash the DNA, following the standard protocol. Measure of DNA concentration of the digestion productsWe used the biophotometer.
List of ligations
Creation of a registry of pFliL, pFlhDC, and FlhDCAnalysis of the transformation we did yesterdayL143, L144, T1 and T2 showed no colonies. The positive control with pUC19 worked well.
Cleaning of the DNA after the digestionWe used the QIAcube to wash the DNA, following the standard protocol. Measure of DNA concentration of the digestion productsWe used the biophotometer.
List of ligations
Construction of pLas-TetR-GFP tripart & rbs-LasR-dble terTransformation
Digestion check from yesterday
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"