Team:Paris/September 4
From 2008.igem.org
(Difference between revisions)
(→PCR Screening of L173 transformants) |
(→Measurement of the concentration of D168 purified) |
||
| (25 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Paris/Calendar_Links|September 3|September 5}} | {{Paris/Calendar_Links|September 3|September 5}} | ||
| - | + | =Construction of rbs-TetR-mRFP-LVA-tripart (L173)= | |
| + | [[Image:Part_icon_rbs.png]][[Image:icon_coding.png]][[Image:Part_icon_reporter.png]][[Image:Part_icon_terminator.png]][[Image:Part_icon_terminator.png]] | ||
| - | =PCR Screening of L173 transformants= | + | ==PCR Screening of L173 transformants== |
| - | ==Transformation results (rbs-TetR in mRFP-LVA-tripart-pSB1A3)== | + | ===Transformation results (rbs-TetR in mRFP-LVA-tripart-pSB1A3)=== |
Ligation '''L173''' | Ligation '''L173''' | ||
| Line 28: | Line 29: | ||
|} | |} | ||
| - | ==PCR Screening== | + | ===PCR Screening=== |
| + | |||
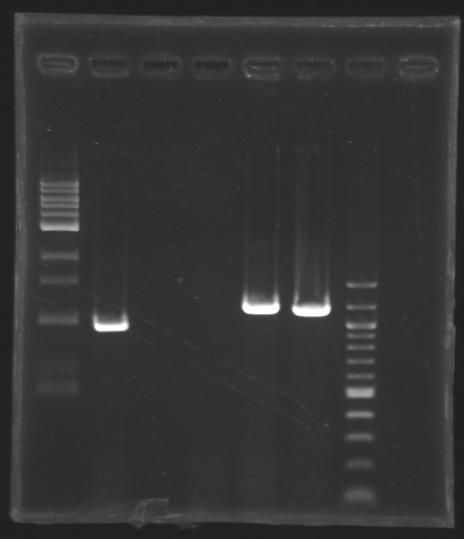
| + | [[Image:KR000292.JPG|thumb|PCR screening]] | ||
PCR screening programm | PCR screening programm | ||
| Line 47: | Line 50: | ||
|- | |- | ||
|'''Sample''' | |'''Sample''' | ||
| - | |1 kb DNA ladder | + | |rowspan="3"|1 kb <br> DNA ladder |
| - | |positive PCR control | + | |rowspan="3"|positive PCR <br> control |
| - | |negative PCR control | + | |rowspan="3"|negative PCR <br> control |
| - | |negative transformation control clone | + | |rowspan="3"|negative transformation <br> control clone |
|L173.1 | |L173.1 | ||
|L173.2 | |L173.2 | ||
| - | |100 bp DNA ladder | + | |rowspan="3"|100 bp <br> DNA ladder |
|- | |- | ||
|'''Expected size''' | |'''Expected size''' | ||
| - | + | |colspan="2"|'''1894 bp''' | |
| - | |colspan="2"|1894 bp | + | |
|- | |- | ||
|'''Measured size''' | |'''Measured size''' | ||
| + | |1,2 kb | ||
| + | |1,2 kb | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | <br>'''Results''': the clones are not correct. | ||
| + | |||
| + | =Digestion= | ||
| + | |||
| + | {|border="1" style="text-align: center" | ||
| + | |'''Digestion n°''' | ||
| + | |'''DNA substrat''' | ||
| + | |'''Digestion by''' | ||
| + | |- | ||
| + | |D112 | ||
| + | |MP106: S03879 (rbs-TetR) in pSB1A2 | ||
| + | |EcoRI & SpeI | ||
| + | |- | ||
| + | |} | ||
| + | <br> | ||
| + | *5 µL of DNA | ||
| + | *3 µL of 10X buffer n°2 | ||
| + | *0,3 µL 100X BSA | ||
| + | *1 µL of EcoRI | ||
| + | *1 µL of SpeI | ||
| + | *19,7 µL of water | ||
| + | <br> | ||
| + | Incubation 2h30 at 37°C and then 20 min at 65°C. | ||
| + | <br>Not purified yet. Put at -20°C. | ||
| + | |||
| + | =Construction of pFlhB-mRFP-LVA-tripart & pFliL-ECFP-LVA-tripart= | ||
| + | [[Image:Part_icon_regulatory.png]][[Image:Part_icon_rbs.png]][[Image:Part_icon_reporter.png]][[Image:Part_icon_terminator.png]][[Image:Part_icon_terminator.png]] | ||
| + | |||
| + | *L183 = pFlhB - mRFP LVA+ | ||
| + | *L184 = pFliL - ECFP LVA+ | ||
| + | *L185 = pFliL - ECFP LVA- | ||
| + | |||
| + | |||
| + | ==Measurement of the concentration of the purified digestion== | ||
| + | [[Team:Paris/Notebook/Protocols#Concentration_of_the_Miniprep | Protocol (it's same that for Miniprep)]] | ||
| + | |||
| + | {|border="1" style="text-align: center" | ||
| + | |'''Digestion Name''' | ||
| + | |'''Concentration (µg/mL)''' | ||
| + | |'''Ratio 260/280''' | ||
| + | |- | ||
| + | |D134.2 | ||
| + | |114 | ||
| + | |nd | ||
| + | |- | ||
| + | |D134.2 | ||
| + | |84,4 | ||
| + | |nd | ||
| + | |- | ||
| + | |D149.2 | ||
| + | |140 | ||
| + | |nd | ||
| + | |} | ||
| + | |||
| + | =>the measurement of the absorbance was so instable, that we have prefered to do it again, <br>we do it by an another thechnik : Analysis on gel. | ||
| + | |||
| + | ==Ligation== | ||
| + | [[Team:Paris/Notebook/Protocols#Ligation |Protocol]] | ||
| + | |||
| + | {|border="1" style="text-align: center" | ||
| + | |'''Ligation Name''' | ||
| + | |'''Vector Name''' | ||
| + | |'''Volume Vector (µL)''' | ||
| + | |'''Insert''' | ||
| + | |'''Volume Insert (µL)''' | ||
| + | |- | ||
| + | |L183 | ||
| + | |D187 | ||
| | | | ||
| + | |D134 | ||
| | | | ||
| + | |- | ||
| + | |L184 | ||
| + | |D198 | ||
| | | | ||
| + | |D149 | ||
| | | | ||
| + | |- | ||
| + | |L185 | ||
| + | |D199 | ||
| | | | ||
| + | |D149 | ||
| | | | ||
| - | |||
| - | |||
|} | |} | ||
| - | = | + | =>it has been missed to do adaptated control. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 19:15, 16 September 2008
Construction of rbs-TetR-mRFP-LVA-tripart (L173)PCR Screening of L173 transformantsTransformation results (rbs-TetR in mRFP-LVA-tripart-pSB1A3)Ligation L173
PCR ScreeningPCR screening programm
Digestion
Construction of pFlhB-mRFP-LVA-tripart & pFliL-ECFP-LVA-tripart
Measurement of the concentration of the purified digestionProtocol (it's same that for Miniprep)
=>the measurement of the absorbance was so instable, that we have prefered to do it again, Ligation
=>it has been missed to do adaptated control. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"