Team:Paris/September 11
From 2008.igem.org
(→Ligation) |
(→Digestion) |
||
| (14 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
[[Image:Before-excision.JPG|thumb|'''Before excision''']] | [[Image:Before-excision.JPG|thumb|'''Before excision''']] | ||
| - | |||
DNA used for digestion: MP101 | DNA used for digestion: MP101 | ||
| Line 71: | Line 70: | ||
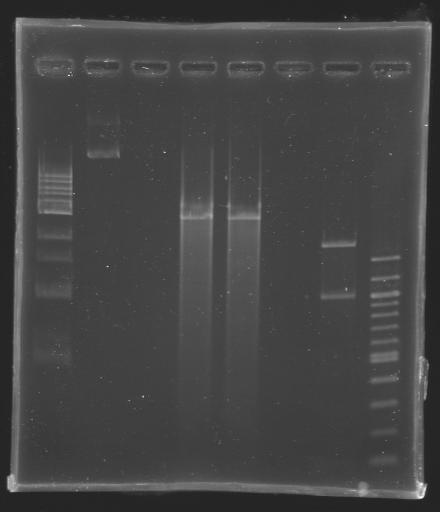
*by gel extraction | *by gel extraction | ||
*or by column | *or by column | ||
| + | <br> | ||
D203 (BspHI digestion) was purified: | D203 (BspHI digestion) was purified: | ||
*by column | *by column | ||
| + | <br> | ||
Elution in 30 µL of EB buffer | Elution in 30 µL of EB buffer | ||
==Ligation== | ==Ligation== | ||
| - | 3 ligases tested | + | '''3 ligases tested''' |
| - | *ligase 1: our 250 µL (400 000 U/mL) tube | + | *ligase '''1''': our 250 µL (400 000 U/mL) tube |
| - | *ligase 2: our 50 µL (400 000 U/mL) tube | + | *ligase '''2''': our 50 µL (400 000 U/mL) tube |
| - | *ligase 3: 2nd floor 50 µL (400 000 U/mL) tube | + | *ligase '''3''': 2nd floor 50 µL (400 000 U/mL) tube |
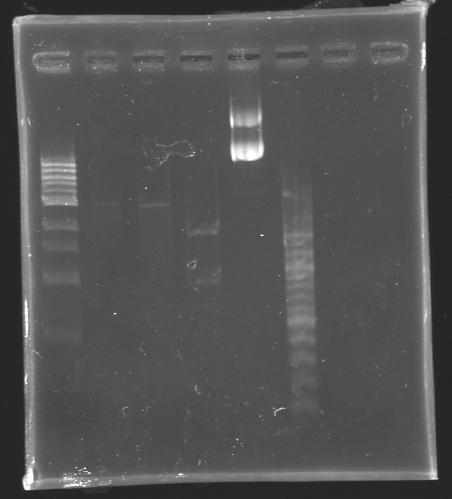
| + | The ligase 1 and 2 have been used with our own buffer tube, whereas the ligase 3 has been used with the buffer tube from the 2nd floor lab. | ||
| + | <br> | ||
| + | <br>'''Reaction mixture''' | ||
| + | *8 µL of purified digestion products | ||
| + | *2 µL of T4 DNA ligase 10X buffer | ||
| + | *9 µL of water | ||
| + | *1 µL of T4 DNA ligase | ||
| + | |||
| + | <br>For the samples for which the digestion products have not been purified, 1 µL of ligase and 1 µL of ATP (1 mM final concentration) have been added directly in the digestion products (in the digestion buffer 2). | ||
| + | <br> | ||
| + | <br>'''Reaction mixture for unpurified digestion products''' | ||
| + | *8 µL of unpurified | ||
| + | *1 µL of ATP 10 mM (1mM final concentration) | ||
| + | *1 µL of T4 DNA ligase | ||
| + | |||
| + | <br>All the ligation were carried out '''overnight''' at '''4°C'''. | ||
{|border="1" style="text-align: center" | {|border="1" style="text-align: center" | ||
Latest revision as of 19:34, 16 September 2008
Checking our ligasesBecause we couldn't be sure of the results of yesterday experiment, we decided to carry it out one more time. DigestionDNA used for digestion: MP101
Reaction mixture
2h30 at 37°C and then 20 min at 65°C
PurificationD202 (EcoRI digestion) was purified in 2 ways:
Ligation3 ligases tested
The ligase 1 and 2 have been used with our own buffer tube, whereas the ligase 3 has been used with the buffer tube from the 2nd floor lab.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"