Team:Paris/August 7
From 2008.igem.org
(Difference between revisions)
| Line 67: | Line 67: | ||
|align="center"|pUC19 | |align="center"|pUC19 | ||
|align="center"|Amp | |align="center"|Amp | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | == '''PCR Screening of Ligation Transformants of 1st August'''== | ||
| + | |||
| + | Use of 8 clones of Ligation transformants for screening PCR | ||
| + | |||
| + | |||
| + | ===Protocol of screening PCR=== | ||
| + | |||
| + | * '''Mix''' | ||
| + | {| Border="1" | ||
| + | |align="center"|'''Name''' | ||
| + | |align="center"|'''Vol (µl)''' | ||
| + | |align="center"|'''Concentration''' | ||
| + | |- | ||
| + | |align="center"|Quick Load | ||
| + | |align="center"|25µl | ||
| + | |align="center"|2X | ||
| + | |- | ||
| + | |align="center"|OligoF_VF2 (O18) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|OligoR_VR (O19) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|water | ||
| + | |align="center"|23µl | ||
| + | |- | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | * 50µl of Mix PCR by tube/clone | ||
| + | * one toothpick of each clone's colony by tube | ||
| + | * Program : Annealing 55°C - Time élongation 1'30" - Number cycle : 29 | ||
| + | |||
| + | |||
| + | === Conditions of electrophoresis === | ||
| + | |||
| + | |||
| + | * 10µl of ladder 1 kb | ||
| + | * 10µl of screening PCR | ||
| + | * migration ~30min at 100W on '''1%''' gel | ||
| + | |||
| + | |||
| + | ===Results=== | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR1_’’’L101(1-8)’’’ | ||
| + | |colspan="3"|PCR2_’’’L102(1-8)’’’ | ||
| + | |colspan="3"|PCR3_’’’L113(1-8)’’’ | ||
| + | |colspan="3"|PCR4_’’’L114(1-8)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
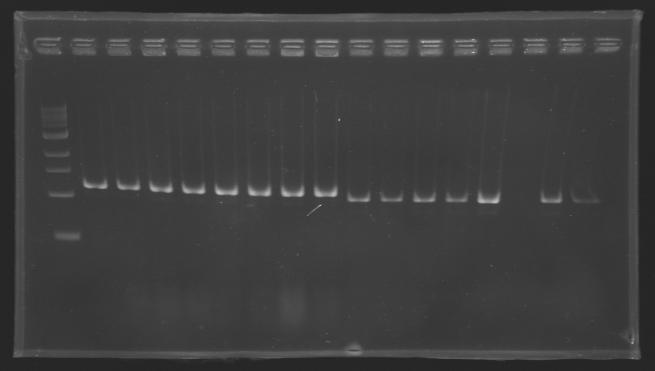
| + | |align="center"| | ||
| + | |align="center"| | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| | ||
| + | |align="center"| | ||
| + | |align="center"|10-->17 | ||
| + | |align="center"| | ||
| + | |align="center"| | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| | ||
| + | |align="center"| | ||
| + | |align="center"|10-->17 | ||
| + | |- | ||
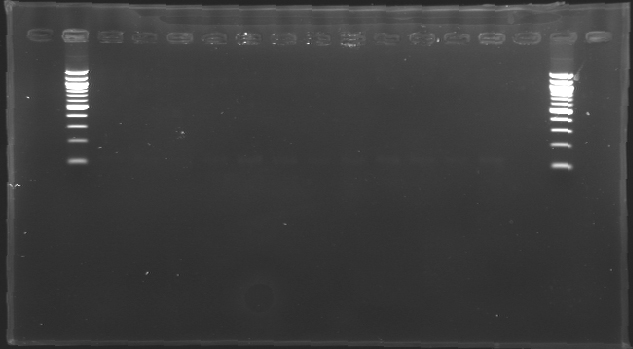
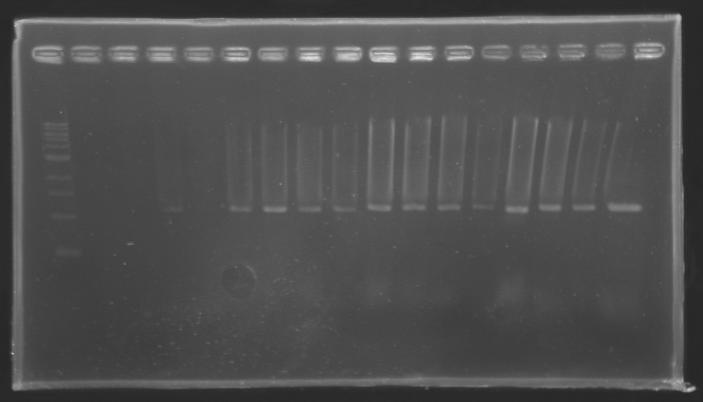
| + | |colspan="6"|[[Image: KR000118_gel1.jpg|thumb|'''Gel 1 : L100-L101''']] | ||
| + | |colspan="6"|[[Image: KR000119_gel2.jpg|thumb|'''Gel 2 : L113-L114''']] | ||
| + | |} | ||
| + | |||
| + | * | ||
| + | {| border="1" | ||
| + | |colspan="3"|PCR5_’’’L106(1-8)’’’ | ||
| + | |colspan="3"|PCR6_’’’L107(1-8)’’’ | ||
| + | |colspan="3"|PCR7_’’’L108.1(1-8)’’’ | ||
| + | |colspan="3"|PCR8_’’’L108.2(1-8)’’’ | ||
| + | |- | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Band''' | ||
| + | |- | ||
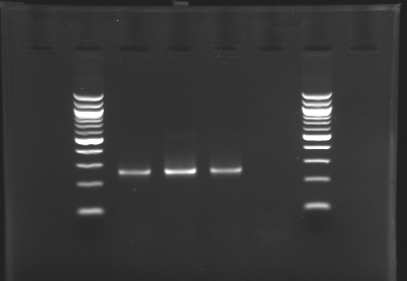
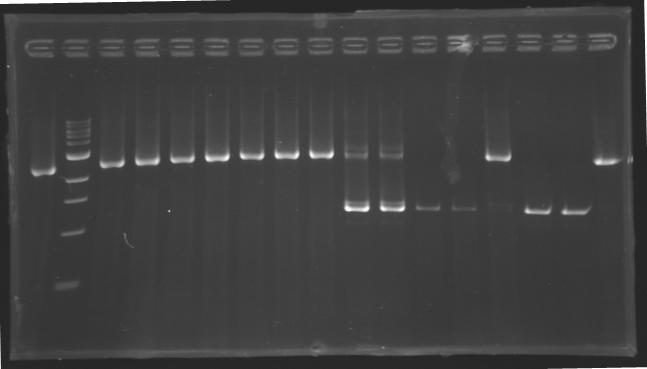
| + | |align="center"| 1200 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| 1239 pb | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"|10-->17 | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|2-->9 | ||
| + | |align="center"| 1200 pb | ||
| + | |align="center"|1200 pb | ||
| + | |align="center"|10-->17 | ||
| + | |- | ||
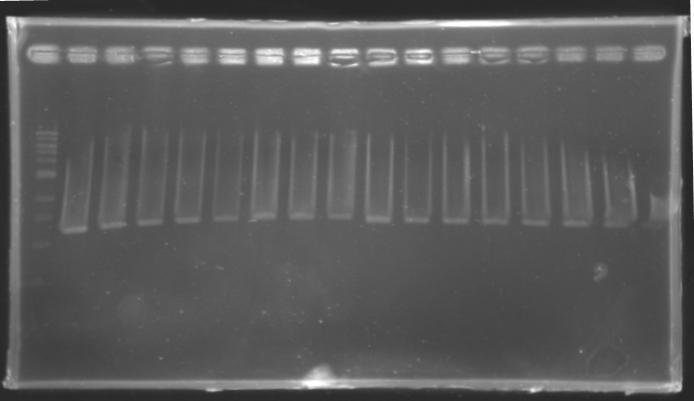
| + | |colspan="6"|[[Image: KR000087_3.jpg|thumb|'''Gel 3 : L106-L107''']] | ||
| + | |colspan="6"|[[Image: KR000089_4.jpg|thumb|'''Gel 4 : L108.1-L108.2''']] | ||
|} | |} | ||
Revision as of 16:26, 7 August 2008
Result of the isolation of coloniesE0240 and pSB3K3 are ok : there is a lot of single colonies S120 and S121 : there is a problem, there is nothing on the plates. We have to check whether those strains are really resistant to Amp. Results of the PCR we did last night
TransformationsProtocolUse of TOP10 chemically competentcells
List of the Ligation Transformation
PCR Screening of Ligation Transformants of 1st AugustUse of 8 clones of Ligation transformants for screening PCR
Protocol of screening PCR
Conditions of electrophoresis
Results
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"