Team:Paris/August 7
From 2008.igem.org
(→Results) |
(→PCR Screening of Ligation Transformants of 1st August) |
||
| Line 321: | Line 321: | ||
===Results=== | ===Results=== | ||
| + | |||
| + | |||
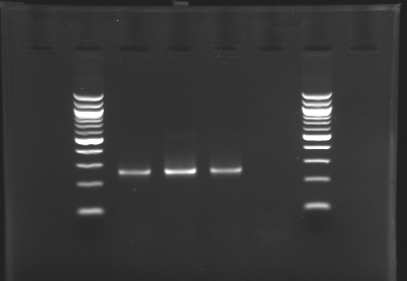
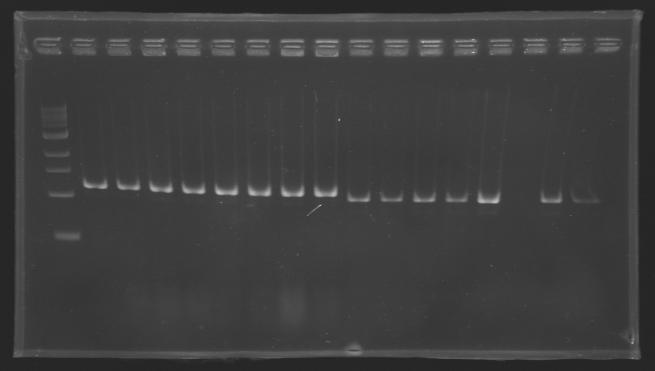
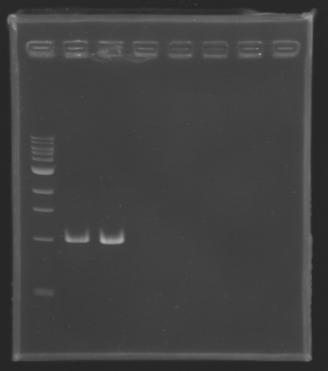
| + | Gel 1 : L100-L101[[Image: KR000118_gel1.jpg|200px]] | ||
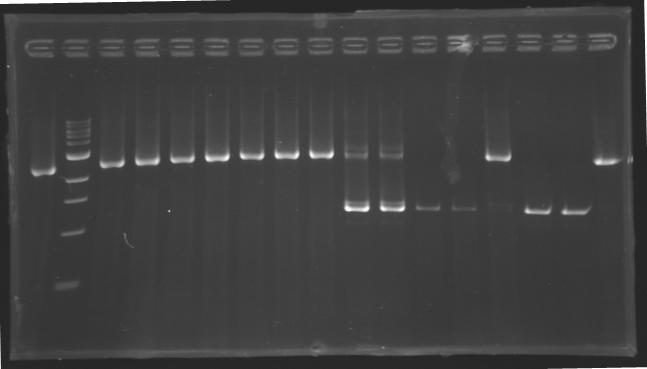
| + | Gel 2 : L113-L114[[Image: KR000119_gel2.jpg|200px]] | ||
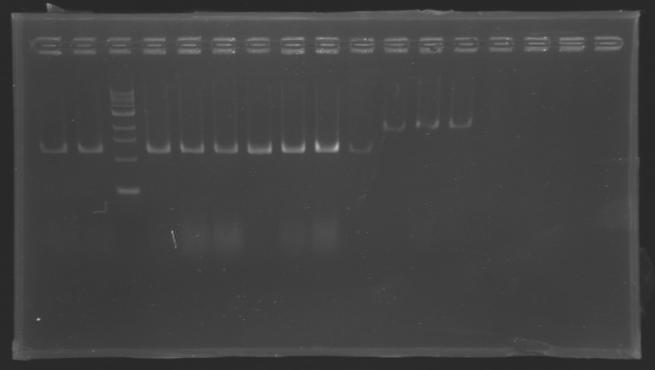
| + | Gel 3 : L120-L122[[Image: KR000120_gel3.jpg|200px]]<br> | ||
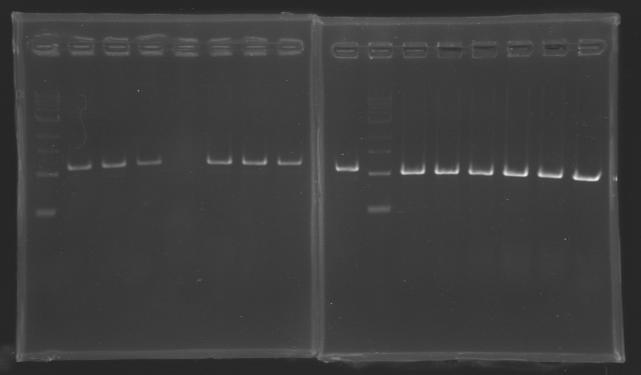
| + | Gel 4 : L123-L126[[Image: KR000122_gel4.jpg|200px]] | ||
| + | Gel 5 : L126[[Image: KR000123_gel5.jpg|100px]] | ||
* | * | ||
{| border="1" | {| border="1" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|align="center"|'''Expected size''' | |align="center"|'''Expected size''' | ||
|align="center"|'''Measured size''' | |align="center"|'''Measured size''' | ||
| + | |align="center"|'''Gel''' | ||
|align="center"|'''Band''' | |align="center"|'''Band''' | ||
|- | |- | ||
| + | |align="center"|PCR1_’’’L101(1-8)’’’ | ||
|align="center"| | |align="center"| | ||
| - | |align="center"| | + | |align="center"|1100 |
| + | |align="center"|1 | ||
|align="center"|2-9 | |align="center"|2-9 | ||
| + | |- | ||
| + | |align="center"|PCR2_’’’L102(1-8)’’’ | ||
|align="center"| | |align="center"| | ||
| - | |align="center"| | + | |align="center"|1000 |
| + | |align="center"|1 | ||
|align="center"|10-17 | |align="center"|10-17 | ||
| + | |- | ||
| + | |align="center"|PCR3_’’’L113(1-8)’’’ | ||
|align="center"| | |align="center"| | ||
| - | |align="center"| | + | |align="center"|2500 |
| + | |align="center"|2 | ||
|align="center"|1, 3-9 | |align="center"|1, 3-9 | ||
| + | |- | ||
| + | |align="center"|PCR4_’’’L114(1-8)’’’ | ||
|align="center"| | |align="center"| | ||
| - | |align="center"| | + | |align="center"|1200 |
| + | |align="center"|2 | ||
|align="center"|10-17 | |align="center"|10-17 | ||
|- | |- | ||
| - | | | + | |align="center"|PCR5_’’’L120(1-8)’’’ |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|align="center"| | |align="center"| | ||
| + | |align="center"|2000 | ||
| + | |align="center"|3 | ||
|align="center"|1,2, 4-9 | |align="center"|1,2, 4-9 | ||
| + | |- | ||
| + | |align="center"|PCR6_’’’L122(1-4)’’’ | ||
|align="center"| | |align="center"| | ||
| - | |align="center"| | + | |align="center"|1200 |
| + | |align="center"|3 | ||
|align="center"|10-13 | |align="center"|10-13 | ||
| + | |- | ||
| + | |align="center"|PCR7_’’’L123(1-8)’’’ | ||
|align="center"| | |align="center"| | ||
| - | |align="center"| | + | |align="center"|1100 |
| + | |align="center"|4 | ||
|align="center"|2-8, 1 | |align="center"|2-8, 1 | ||
| + | |- | ||
| + | |align="center"|PCR8_’’’L126(1-6)’’’ | ||
|align="center"| | |align="center"| | ||
| - | |align="center"| | + | |align="center"|1000 |
| + | |align="center"|4' | ||
|align="center"|3-8 | |align="center"|3-8 | ||
|- | |- | ||
| - | | | + | |align="center"|PCR5_’’’L126(7-8)’’’ |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|align="center"| | |align="center"| | ||
| + | |align="center"|1000 | ||
| + | |align="center"|6 | ||
|align="center"|2-3 | |align="center"|2-3 | ||
| - | |||
| - | |||
|} | |} | ||
==> '''Conclusion :''' | ==> '''Conclusion :''' | ||
Revision as of 12:40, 8 August 2008
Glycerol Stocks
Result of the isolation of coloniesE0240 and pSB3K3E0240 and pSB3K3 are ok : there is a lot of single colonies S120 and S121S120 and S121 : there is a problem, there is nothing on the plates. We have to check whether those strains are really resistant to Amp.
Preparation of the newly ammplified promotersElectrophoresis of the PCR products made yesterdayElectrophoresis settings
Washing of the PCR products
DNA concentration measurementWe used two methods: With a Spectrophotometer
With a Biophotometer
Remarks :
DigestionProtocol
Digestion mix:
We pu the digestion mix to incubate during 2h at 37°C Results
Conclusion : The digestion of MP123 into D136 worked very well. Tomorrow we will isolate it. We see nothing on the other digestion. Maybe someone forgot to put the template DNA inside the tube !? Anyway, we will do the manipulation again tomorrow. TransformationsProtocolUse of TOP10 chemically competentcells
List of the Ligation Transformation
PCR Screening of Ligation Transformants of 1st AugustUse of 8 clones of Ligation transformants for screening PCR
Protocol of screening PCR
Conditions of electrophoresis
ResultsGel 1 : L100-L101
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"