From 2008.igem.org
(Difference between revisions)
|
|
| Line 578: |
Line 578: |
| | | | | | |
| | |1 | | |1 |
| - | |
| |
| | | | | | |
| | | | | | |
| Line 599: |
Line 598: |
| | | | | | |
| | |3 | | |3 |
| - | |
| |
| | | | | | |
| | | | | | |
Revision as of 17:24, 25 August 2008
|
← Yesterday ↓ Calendar ↑Tomorrow →
Screening of the cloning of E0240 and FlhDC+promotor
Spreading the clones in order to obtain single colonies
| Strain
| Resistance
| Ligation
| DNA cloned
| vector
| expected size of the fragment amplified by VF & VR
| mesured size
|
| S159.1
| kanamycine
| L139.1
| E0240 (GFP tripart)
| pSB3K3
| 1192 bp
| 1,5 kb
1,1 kb
0,6 kb
|
| S161.1
| ampicilline
| L142.7
| FlhDC+promotor
| pSB1A2
| 1403 bp
| 1,4
0,4 kb
0,3 kb
|
The plates obtained from the speading of yesterday can't be used because there are not single colonies.
We have to try again, but with a stronger dilution of the bacteria or with a smaller volume of spreading.
- Resuspension of some bacteria from the glycerol stock into 1 mL of LB+antibiotic
- Dilution 10 and dilution 100
- Spreading of 100 µL of each dilution on a LB plate containing the right antibiotic
- Overnight incubation (37°C)
Miniprep and stock glycerol
Stock Glycerol of New Biobrick
| Stock number
| Biobricks
| Description
|
| S163.1
| B0032
| 
RBS
|
| S163.2
|
| S164.1
| E0422
|    
RBS+ ECFP+ LVA+ term
|
| S164.2
|
| S165.1
| E0430
|    
RBS+ YFP+ LVA- term
|
| S165.2
|
| S166.1
| E0432
|    
RBS+ YFP LVA+ term
|
| S166.2
|
| S167.1
| E0420
|    
RBS+ ECFP LVA- term
|
| S167.2
|
| S168.1
| I732078
|    
RBS+ mRFP LVA+ term
|
| S168.2
|
Miniprep of New Biobricks
| Miniprep
| Biobricks
| Description
|
| MP163.1
| B0032
| 
RBS
|
| MP163.2
|
| MP164.1
| E0422
|    
RBS+ ECFP+ LVA+ term
|
| MP164.2
|
| MP165.1
| E0430
|    
RBS+ YFP+ LVA- term
|
| MP165.2
|
| MP166.1
| E0432
|    
RBS+ YFP LVA+ term
|
| MP166.2
|
| MP167.1
| E0420
|    
RBS+ ECFP LVA- term
|
| MP167.2
|
| MP168.1
| I732078
|    
RBS+ mRFP LVA+ term
|
| MP168.2
|
Construction for FIFO
Aim : Construction of pFlgA - YFP tripart (+/- LVA) "pFlgA-RBS-YFP-dbl ter" (pFlgA-E0430/E0432)     
Digestion
Measurement of concentration of minipreps
standard protocol
| Miniprep
| Biobrick
| C° (µg/mL)
| ratio 260/280
|
| MP164.1
| E0422
| 95
| 1.69
|
| MP164.2
| E422
| 90
| 1.76
|
| MP165.1
| E0430
| 131
| 1.74
|
| MP165.2
| E0430
| nd
| nd
|
| MP166.1
| E0432
| 112
| 1.65
|
| MP166.2
| E0432
| 79
| 1.6
|
| MP167.1
| E0420
| 199
| 1.72
|
| MP167.2
| E0420
| 194
| 1.73
|
| MP168.1
| I732078
| 111
| 1.65
|
| MP168.2
| I732078
| 104
| 1.67
|
| MP122.1
| E0840
| 56
| 1.58
|
| MP122.2
| E0840
| 98
| 1.63
|
Digestion
Protocol Digestion
| Name
| Template DNA
| Description
| Vol MP (µl)
| Vol H2O (µl)
| Enzymes
|
| D166
| MP165.1
| RBS+ YFP LVA- term - FV
| 7.63
| 17
| EcoRI and XbaI
|
| D167
| MP166.1
| RBS+ YFP LVA+ term - FV
| 8.9
| 15.8
| EcoRI and XbaI
|
| D131
| MP122.2
| GFP tripart - I
| 10.2
| 14.5
| XbaI and PstI
|
Protocol
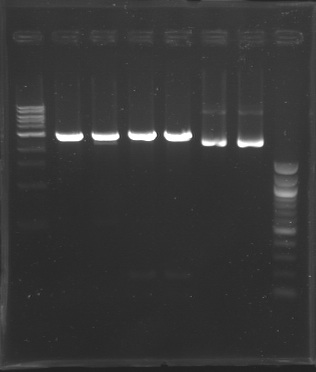
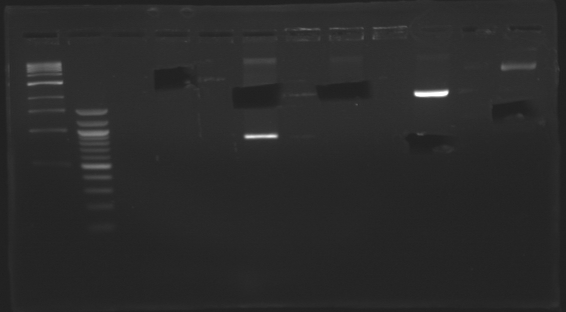
 Gel Extraction of D166-D167-D131
| Well
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
| 9
| 10
| 11
| 12
|
| Sample
| 1kb ladder
| MP165.1
| MP166.1
| MP122.2
| no sample
| D166
| no sample
| D167
| no sample
| D131
| no sample
| 100pb ladder
|
| Expected size (pb)
|
| 2 957
| 2 996
| 2 957
|
| 2942
|
| 914
|
| 900
|
| Measured size (pb)
|
| 3 000
| 3 000
| 3 000
|
| 2500
|
| 2500
|
| 950
|
|
|
=> Following a mistake, the right E0430(D166) and E0432(D167) digestion, have not been purified.
So we need to repeat the same digestion experiment tomorrow morning.
Results of the transformation we did yesterday
Number of colonies
| Ligation name
| Description
| Antibio
| Number Colonies observed
| Fluorescence
| Comments
|
| Ligations
|
| L153
| D123(BV) - D165(BI)
J23100 - rbs-LasR-Double terminator
| Amp
| 768
| No
| OK
|
| L154
| D103(BV) - D165(FV)
J23101 - rbs-LasR - Double terminator
| Amp
| 236
| No
| OK
|
| Controls
|
| C1
| D123(BV)
| Amp
| 23
| No
| OK
|
| C2
| D103(FV)
| Amp
| 144
| No
| OK
|
| Positive Control
| pUC19
| Amp
| 2264 (efficiency 4,5.10^8)
| No
| OK
|
PCR Screening
==> Protocol
 Ligations results J23100+D165 and J23101+D165
| Well
| Sample
| Expected size
| Measured size
|
| 1
| 153.1
| 1194
| 1200
|
| 2
| 153.2
|
| 3
| 153.3
|
| 4
| L153.4
|
| 5
| L153.5
|
| 6
| L153.6
|
| 7
| L153.7
|
| 8
| L153.8
|
| 9
| 1kb ladder
|
|
|
| 10
| L154.1
| 1194
| 1200
|
| 11
| L154.2
|
| 12
| L154.3
|
| 13
| L154.4
|
| 14
| L154.5
|
| 15
| L154.6
|
| 16
| L154.7
|
| 17
| L154.8
|
Minipreps
| Miniprep Name
| Ligation name
| Antibio
| Biobricks
| Description
|
| MP169.1
| L153.1
| Amp
|     
| J23100 - rbs-LasR-Double terminator
|
| MP169.2
| L153.2
|
| MP170.1
| L154.1
|     
| J23101 - rbs-LasR - Double terminator
|
| MP170.2
| L154.2
|
Promoter characterization plasmids
Ligation
Our ligations from yesterday didn't work. The positive control for transformation worked.
Digestion
We had a problem with a gel extraction so we have to make again the digestions from yesterday
Other digestions made:
Protocol Digestion
| Digestion name
| Plasmid
| Description
| Miniprep used
| Enzymes
| Concentration after gel extraction
|
| D179
| MP3.4
| B0015 (double terminator B0010-B0012) - BV
| 4
| SpeI and PstI
| 9
|
| D180
| MP101.1
| promoter J23101- BV
| 1
| SpeI and PstI
| 7
|
| D181
| MP104.2
| PTet (TetR repressible promoter) - FV
| 1
| EcoRI and XbaI
| 1
|
| D182
| MP114.1
| TetR - BI
| 1
| XbaI and PstI
| 10
|
| D183
| MP119.3
| pBad promoter - BI
| 1
| XbaI and PstI
| 0
|
| D184
| MP143.1
| gfp generator - FI
| 2
| EcoRI and SpeI
| 13
|
| D185
| MP163.1
| B0032 RBS - BV
| 2
| SpeI and PstI
| 21
|
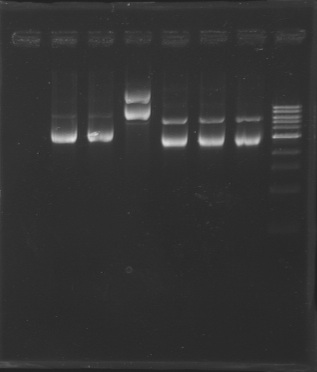
D179
D180
D181
D182
D183
D184
D185
Ligation
Protocol
| Ligation name
| Vector digestion
| Vector description
| Vector volume
| Insert digestion
| Insert description
| Insert volume
| Product description
| Antibiotic
|
| L155
| D164
| J23101 promoter
| 10
| D163
| gfp generator
| 2
| J23101 promoter-gfp generator
| Amp
|
| L156
| D161
| pTet promoter
| 1
| D163
| gfp generator
| 4
| pTet promoter-gfp generator
| Kana
|
|
| D161
|
| 1
|
|
|
| Vector autoligation control
| Kana
|
| L157
| D125.2
| B0015
| 3
| D162
| 4
| tetR
| tetR-B0015
| Amp
|
|
| D125.2
|
| 3
|
|
|
| Vector autoligation control
| Amp
|
Sequencing
- We received results of our sequencing from COCHIN
- We succeed for FlgA promoter
- FlgB and flhB don't match with our expected sequences. We decided to cut our Miniprep product with other enzymes to check our sequence.
Digestion
Protocol
- D176 : pFlgB digested with ApoI
- D177 : pFlgB digested with NruI
- D178 : pFlhB digested with BstAPI
Screening
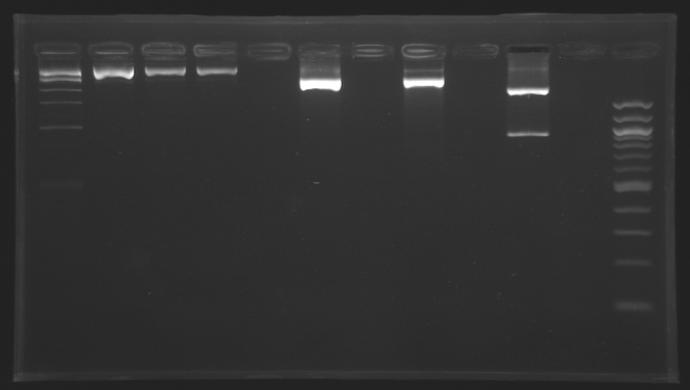
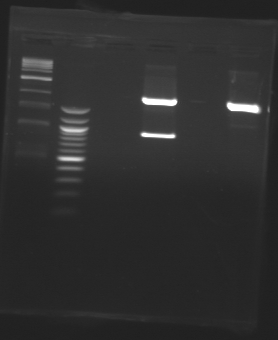
Gel 1
| Well
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
|
| Sample
| 1kb ladder
| D176.1
| D176.2
| D176.3
| D176.4
| D177.1
| D177.2
| 100pb ladder
|
| Expected size
|
| 2970
194
48
| 3212
|
| Measured size
|
| 2900
200
/
| Plamsid not digested
|
|
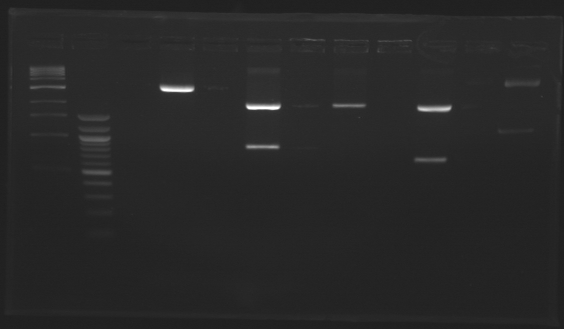
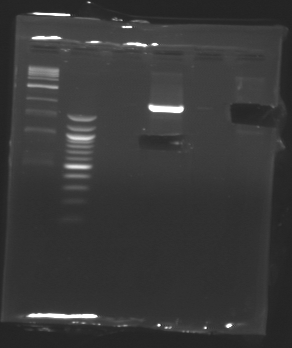
Gel 2
| Well
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
|
| Sample
| nothing
| D177.3
| D177.4
| D178.1
| D178.2
| D178.3
| D178.4
| 1kb ladder
|
| Expected size
|
| 3212
| 3213
|
| Measured size
|
| Plamsid not digested
|
|
Conclusion => the sequence of pFlgB and pFlhB are not good we will tried to isolate pFlhB again.
|
 "
"