Team:Paris/August 8
From 2008.igem.org
(Difference between revisions)
(→creening PCR of transformants) |
|||
| Line 74: | Line 74: | ||
|} | |} | ||
| - | == | + | == '''PCR Screening of Ligation Transformants'''== |
| + | |||
| + | Use of 8 clones of Ligation transformants for screening PCR | ||
| + | |||
| + | | Border="1" | ||
| + | |align="center"|'''Ligation''' | ||
| + | |align="center"|'''Name''' | ||
| + | |align="center"|'''n° clone''' | ||
| + | |align="center"|'''fluorescence''' | ||
| + | |- | ||
| + | |align="center"|L128 | ||
| + | |align="center"|pFlgA | ||
| + | |align="center"| | ||
| + | |- | ||
| + | |align="center"|OligoF_VF2 (O18) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|OligoR_VR (O19) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|water | ||
| + | |align="center"|23µl | ||
| + | |- | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | ===Protocol of screening PCR=== | ||
| + | |||
| + | * '''Mix''' | ||
| + | {| Border="1" | ||
| + | |align="center"|'''Name''' | ||
| + | |align="center"|'''Vol (µl)''' | ||
| + | |align="center"|'''Concentration''' | ||
| + | |- | ||
| + | |align="center"|Quick Load | ||
| + | |align="center"|25µl | ||
| + | |align="center"|2X | ||
| + | |- | ||
| + | |align="center"|OligoF_VF2 (O18) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|OligoR_VR (O19) | ||
| + | |align="center"|1µl | ||
| + | |align="center"|10µM | ||
| + | |- | ||
| + | |align="center"|water | ||
| + | |align="center"|23µl | ||
| + | |- | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | * 50µl of Mix PCR by tube/clone | ||
| + | * one toothpick of each clone's colony by tube | ||
| + | * Program : Annealing 55°C - Time élongation 1'30" - Number cycle : 29 | ||
| + | |||
| + | |||
| + | === Conditions of electrophoresis === | ||
| + | |||
| + | |||
| + | * 10µl of ladder 100 pb | ||
| + | * 10µl of screening PCR | ||
| + | * migration ~30min at 100W on '''1,5%''' gel | ||
| + | |||
| + | |||
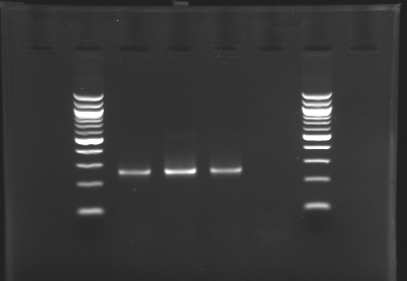
| + | ===Results for L100=== | ||
| + | |||
| + | L100= D110 + D130 = '''RBS-tetR-ECFP-Ter''' [[Image:Part_icon_rbs.png]][[Image:Icon_coding.png]][[Image:Part_icon_rbs.png]][[Image:Part_icon_reporter.png]][[Image:Part_icon_terminator.png]] | ||
| + | |||
==Preparation of the newly ammplified promoters== | ==Preparation of the newly ammplified promoters== | ||
===Electrophoresis of the PCR products made [[Team:Paris/August_6|yesterday]]=== | ===Electrophoresis of the PCR products made [[Team:Paris/August_6|yesterday]]=== | ||
Revision as of 09:47, 11 August 2008
|
Minipreps : Plasmid extractionExtraction of pSB3K3 et E0240 in pSB1A2 plasmid from overnight bacteria culture using the QIAspin Miniprep Kit (QIAGEN) by QIACube.
Transformation results
PCR Screening of Ligation TransformantsUse of 8 clones of Ligation transformants for screening PCR | Border="1" | Ligation | Name | n° clone | fluorescence | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| L128 | pFlgA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OligoF_VF2 (O18) | 1µl | 10µM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OligoR_VR (O19) | 1µl | 10µM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| water | 23µl | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Protocol of screening PCR
- Mix
| Name | Vol (µl) | Concentration |
| Quick Load | 25µl | 2X |
| OligoF_VF2 (O18) | 1µl | 10µM |
| OligoR_VR (O19) | 1µl | 10µM |
| water | 23µl |
- 50µl of Mix PCR by tube/clone
- one toothpick of each clone's colony by tube
- Program : Annealing 55°C - Time élongation 1'30" - Number cycle : 29
Conditions of electrophoresis
- 10µl of ladder 100 pb
- 10µl of screening PCR
- migration ~30min at 100W on 1,5% gel
Results for L100
L100= D110 + D130 = RBS-tetR-ECFP-Ter ![]()
![]()
![]()
![]()
![]()
Preparation of the newly ammplified promoters
Electrophoresis of the PCR products made yesterday
Electrophoresis settings
- Gel : 1.5 % agar
- 3µL template DNA
- 10µL QuickLoad DNA ladder 100 bp
| Name | Promotor | Gel | Band | Expected size | Measured size |
| PCR_124 | pFlgA | 1 | 2 | 250 pb | |
| PCR_125 | pFlgB | 1 | 3 | 250 pb | |
| PCR_126 | pFlhB | 1 | 4 | 250 pb | |
| PCR_127 | pFlhDC | 2 | 2 to 13 | nothing |
Results
- We have no results for pflhDC, wo don't know yet where is the problem. We will try with other conditions! (yet undetermined)
- Concerning pflhB, pflgA and pflgB, the protocol seems to be very operational: we always have great results !
 "
"