|
|
| Line 211: |
Line 211: |
| | |1.84 | | |1.84 |
| | |} | | |} |
| - |
| |
| - | ==Transformation results==
| |
| - |
| |
| - | {| border="1"
| |
| - | |align="center"|'''Name'''
| |
| - | |align="center"|'''Description'''
| |
| - | |align="center"|'''Antibio'''
| |
| - | |align="center"|'''Number of colonies'''
| |
| - | |align="center"|'''Number of red fluorescent colonies'''
| |
| - | |-
| |
| - | |colspan="5"|'''Ligation '''
| |
| - | |-
| |
| - | |align="center"|L128
| |
| - | |align="center"|J61002-pFlgA<br>D136 (FV) - D132 (FI)
| |
| - | |align="center"|Amp
| |
| - | |align="center"|~ 400
| |
| - | |align="center"|2
| |
| - | |-
| |
| - | |align="center"|L129
| |
| - | |align="center"|J61002-pFlgB<br>D136 (FV) - D133 (FI)
| |
| - | |align="center"|Amp
| |
| - | |align="center"|39
| |
| - | |align="center"|5
| |
| - | |-
| |
| - | |align="center"|L130
| |
| - | |align="center"|J61002-pFlhB<br>D136 (FV) - D134 (FI)
| |
| - | |align="center"|Amp
| |
| - | |align="center"|~ 1000
| |
| - | |align="center"|4 (but 3 are on the edge of the petri dishe)
| |
| - | |-
| |
| - | |align="center"|L131
| |
| - | |align="center"|J61002-pFlhDC<br>D136 (FV) - D135 (FI)
| |
| - | |align="center"|Amp
| |
| - | |align="center"|39
| |
| - | |align="center"|38
| |
| - | |-
| |
| - | |colspan="5"|'''Control '''
| |
| - | |-
| |
| - | |align="center"|Control 1
| |
| - | |align="center"|D136
| |
| - | |align="center"|Amp
| |
| - | |align="center"|0
| |
| - | |align="center"|0
| |
| - | |-
| |
| - | |align="center"|Positive control
| |
| - | |align="center"|pUC19
| |
| - | |align="center"|Amp
| |
| - | |align="center"|36
| |
| - | |align="center"|0
| |
| - | |}
| |
| - |
| |
| - | == '''PCR Screening of Ligation Transformants'''==
| |
| - |
| |
| - | Use of 8 clones of Ligation transformants for screening PCR
| |
| - |
| |
| - |
| |
| - | {|| Border="1"
| |
| - | |align="center"|'''Ligation'''
| |
| - | |align="center"|'''Name'''
| |
| - | |align="center"|'''n° clone'''
| |
| - | |align="center"|'''fluorescence'''
| |
| - | |-
| |
| - | | rowspan="8" align="center"|L128
| |
| - | | rowspan="8" align="center"|pFlgA
| |
| - | |align="center"|1
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|2
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|3
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|4
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|5
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|6
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|7
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|8
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | | rowspan="8" align="center"|L129
| |
| - | | rowspan="8" align="center"|pFlgB
| |
| - | |align="center"|1
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|2
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|3
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|4
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|5
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|6
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|7
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|8
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | | rowspan="8" align="center"|L130
| |
| - | | rowspan="8" align="center"|pFlhB
| |
| - | |align="center"|1
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|2
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|3
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|4
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|5
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|6
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|7
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |align="center"|8
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | | rowspan="8" align="center"|L131
| |
| - | | rowspan="8" align="center"|pFlgB
| |
| - | |align="center"|1
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|2
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|3
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|4
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|5
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|6
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|7
| |
| - | |align="center"|red
| |
| - | |-
| |
| - | |align="center"|8
| |
| - | |align="center"|no
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - |
| |
| - |
| |
| - | ===Protocol of screening PCR===
| |
| - |
| |
| - | * '''Mix'''
| |
| - | {| Border="1"
| |
| - | |align="center"|'''Name'''
| |
| - | |align="center"|'''Vol (µl)'''
| |
| - | |align="center"|'''Concentration'''
| |
| - | |-
| |
| - | |align="center"|Quick Load
| |
| - | |align="center"|25µl
| |
| - | |align="center"|2X
| |
| - | |-
| |
| - | |align="center"|OligoF_VF2 (O18)
| |
| - | |align="center"|1µl
| |
| - | |align="center"|10µM
| |
| - | |-
| |
| - | |align="center"|OligoR_VR (O19)
| |
| - | |align="center"|1µl
| |
| - | |align="center"|10µM
| |
| - | |-
| |
| - | |align="center"|water
| |
| - | |align="center"|23µl
| |
| - | |-
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | * 50µl of Mix PCR by tube/clone
| |
| - | * one toothpick of each clone's colony by tube
| |
| - | * Program : Annealing 55°C - Time élongation 1'30" - Number cycle : 29
| |
| - |
| |
| - |
| |
| - | === Conditions of electrophoresis ===
| |
| - |
| |
| - |
| |
| - | * 10µl of ladder 100 pb
| |
| - | * 10µl of screening PCR
| |
| - | * migration ~30min at 100W on '''1,5%''' gel
| |
| - |
| |
| - |
| |
| - | * Program PCR "Screening": Annealing 55°C - Time élongation 1'30" - Number cycle : 29
| |
| - |
| |
| - |
| |
| - |
| |
| - | === Electrophoresis Purification of PCR===
| |
| - |
| |
| - | ''When the PCR cycles were finished,''
| |
| - |
| |
| - | '''conditions :'''
| |
| - |
| |
| - | * 10µl of ladder 1 kb (unlike 100 pb)
| |
| - | * 2 x 30µl of PCR products added with 10µl of loading Dye 6x
| |
| - | * migration ~30min at 100W on a '''1,5% agarose gel'''.
| |
| - |
| |
| - |
| |
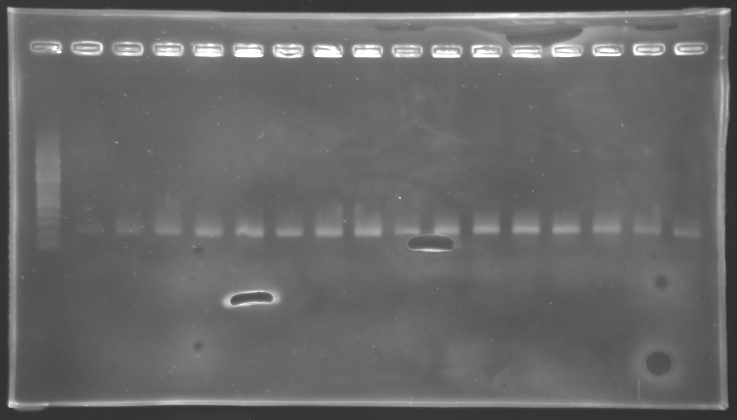
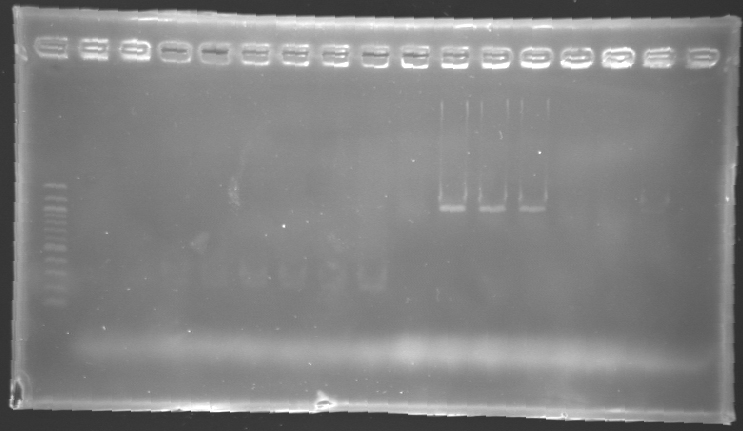
| - | '''Results of electrophoresis'''
| |
| - |
| |
| - | <br>gel 1 [[Image:KR000143.jpg | gel 1|200px]]
| |
| - | gel 2 [[Image:KR000145.jpg|gel 2|200px]]
| |
| - |
| |
| - | {|- border="1"
| |
| - | |align="center"|'''Name'''
| |
| - | |align="center"|'''Promotor'''
| |
| - | |align="center"|'''Gel'''
| |
| - | |align="center"|'''Band'''
| |
| - | |align="center"|'''Expected size'''
| |
| - | |align="center"|'''Measured size'''
| |
| - | |-
| |
| - | |align="center"|PCR_124'
| |
| - | |align="center"|pFlgA
| |
| - | |align="center"|1
| |
| - | |align="center"|2 to 9
| |
| - | |style="background: #cbff7B"|<center>261 pb</center>
| |
| - | |align="center"|300 pb
| |
| - | |-
| |
| - | |align="center"|PCR_125'
| |
| - | |align="center"|pFlgB
| |
| - | |align="center"|1
| |
| - | |align="center"|10 to 17
| |
| - | |style="background: #cbff7B"|<center>261 pb</center>
| |
| - | |align="center"|300 pb
| |
| - | |-
| |
| - | |align="center"|PCR_126'
| |
| - | |align="center"|pFlhB
| |
| - | |align="center"|2
| |
| - | |align="center"|2 to 9
| |
| - | |style="background: #cbff7B"|<center>260 pb</center>
| |
| - | |align="center"|300 pb
| |
| - | |-
| |
| - | |align="center"|PCR_127'
| |
| - | |align="center"|pFlhDC
| |
| - | |align="center"|2
| |
| - | |align="center"|10 to 17
| |
| - | |style="background: #ff6d73"|<center>446 pb</center>
| |
| - | |align="center"|1,000 pb
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | ==> '''Conclusion:'''
| |
| - | *PCR of pFlgA, pFlgB and pFlhB have succeed, but we always have a problem with pFlhDC probably because of the oligos wich are not specific.
| |
| | | | |
| | ==Transformation results== | | ==Transformation results== |
 "
"