Team:Paris/August 15
From 2008.igem.org
(Difference between revisions)
(→Results) |
(→Digestions) |
||
| Line 148: | Line 148: | ||
=='''Digestions'''== | =='''Digestions'''== | ||
| + | After having succeded in amplifying the promoter and the gene of flhDC, we decided to clone it into a plasmid. | ||
| + | The first step is the digestion. | ||
| + | |||
| + | ==='''Protocol'''=== | ||
| + | {| Border="2" | ||
| + | |align="center"|'''Digestion name''' | ||
| + | |align="center"|'''Template DNA''' | ||
| + | |align="center"|''' Enzymes ''' | ||
| + | |align="center"|'''Volume of DNA''' | ||
| + | |- | ||
| + | |align="center"|D 140 | ||
| + | |align="center"|PCR 130 - E0240 RBS + | ||
| + | |align="center"|PstI | ||
| + | |align="center"|5 µL | ||
| + | |- | ||
| + | |align="center"|D 141 | ||
| + | |align="center"|PCR 131 - flhD rbs - | ||
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
| + | |- | ||
| + | |align="center"|D 142 | ||
| + | |align="center"|PCR 132 - flhC rbs - | ||
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
| + | |- | ||
| + | |align="center"|D 143 | ||
| + | |align="center"|PCR 133 - flhDC + prom | ||
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
| + | |- | ||
| + | |align="center"|D 144 | ||
| + | |align="center"|MP 142 - pSB3K3 | ||
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
| + | |- | ||
| + | |align="center"|D 145 | ||
| + | |align="center"|MP122 - pSB1A2 | ||
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|5 µL | ||
| + | |} | ||
| + | |||
| + | * X µL of Template DNA | ||
| + | * Buffer (n°2) 10X : 3µL | ||
| + | * BSA 100X : 0.3µL | ||
| + | * Pure water qsp 30 µL | ||
| + | * 1 µL of each enzyme | ||
| + | |||
| + | * Incubate during about 3h at 37°C, then 20 minutes at 65°C (to inactivate the enzymes). | ||
| + | * Exclusively for D 144 : 2 µL of Antarctic Phosphatase was added, 30 min at 37°C then 5 min at 65°C. | ||
=='''Digestion'''== | =='''Digestion'''== | ||
Revision as of 18:14, 16 August 2008
Transformation of the ligations we did yesterdayWe transformed L 143, L 144 and the negative controls T1 and T2, using Invitrogen's TOP10 chemically competent cells standard protocol. PCR amplification of flhDC and its promoterList of PCRs
ProtocolWe followed the standard protocol of amplification in Two steps. PCR program used : PHUSION2 ResultsSettings Gel 1%
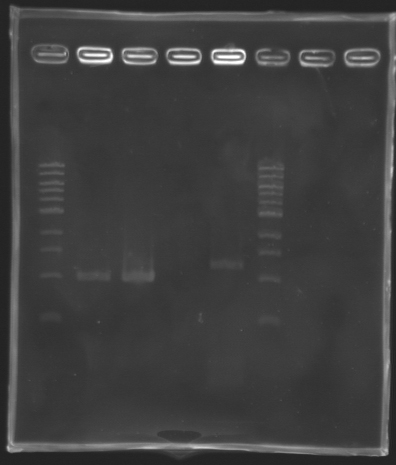
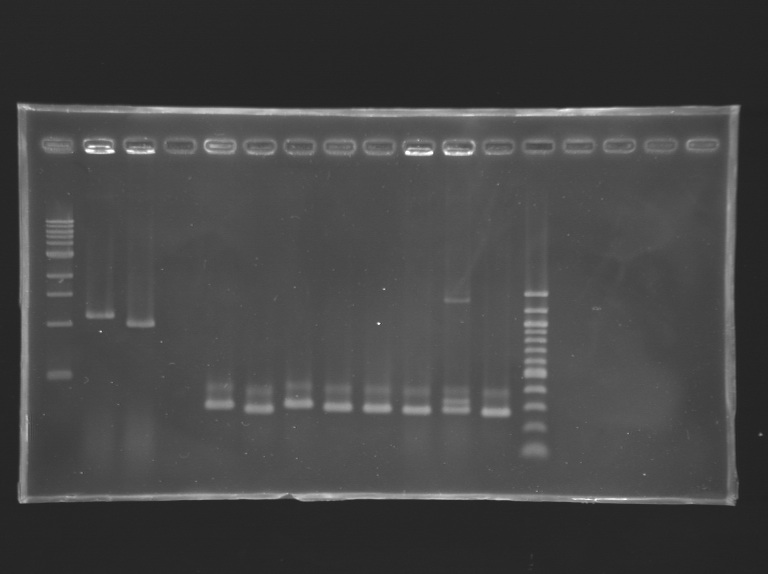
We managed to amplify flhDC ! Settings Gel 2%
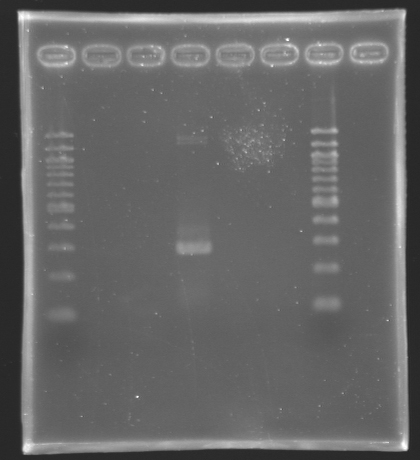
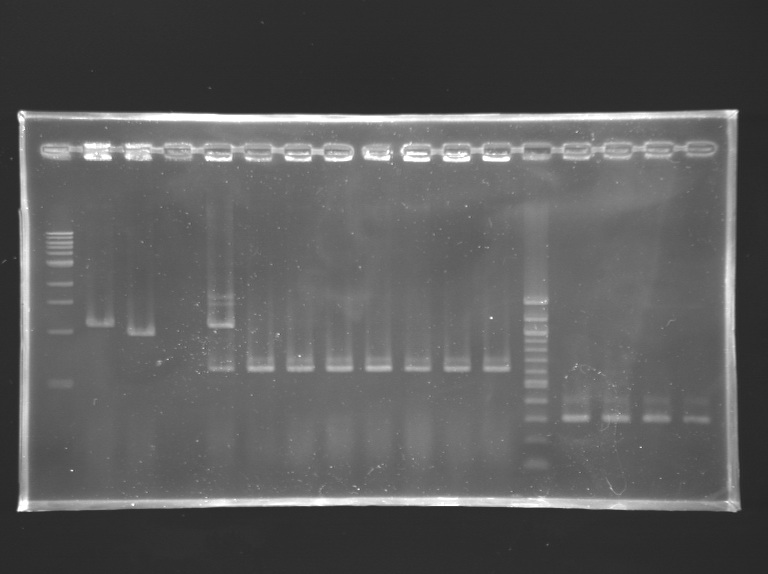
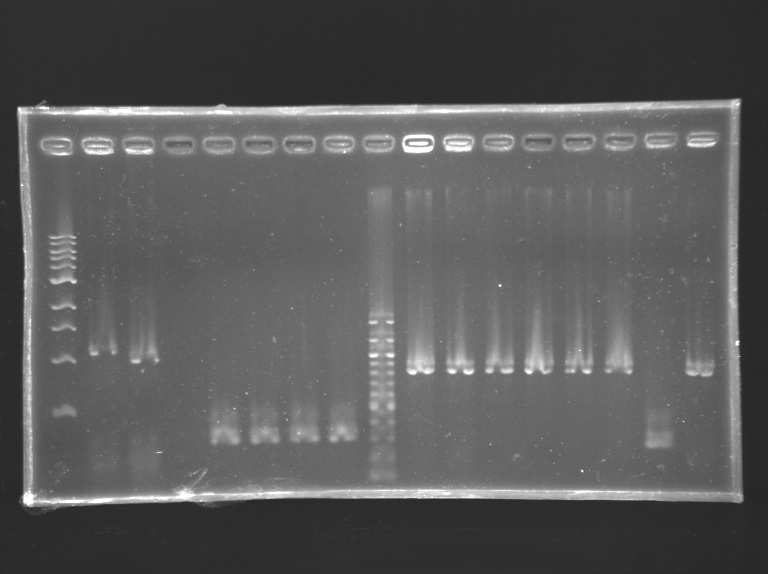
We managed to amplify the promoter of flhDC DigestionsAfter having succeded in amplifying the promoter and the gene of flhDC, we decided to clone it into a plasmid. The first step is the digestion. Protocol
DigestionMeasurement of concentration of minipreps
DigestionLigation
Analysis of yesterday PCR screening
Starting the construction of the Promoter characterization plasmidDigestionMeasurement of concentration of minipreps
Digestion
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"