|
← Yesterday ↓ Calendar ↑Tomorrow →
Transformation
Digestion
PCR
We performed PCR on to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.
PCR amplification
Protocol
| Number
| Name
| Sequence
| Length
| Comments
|
| O110
| FlhDC-Uri-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGAGTTGTATGTGCGTGTAGTGACGAGTACAG
| 58
| Don't amplify the both OmpR binding site
|
| O111
| FlhDC-Total-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGAGTCATTTTTGCTTGCTAGCGTACGGAAAA
| 58
| Amplify the both OmpR binding site
|
| O113
| FlhDC(nu)-R
| GTTTCTTCCTGCAGCGGCCGCTACTAGTACAGAATAACCAACTTTATTTTTATG
| 54
| Don't amplify the natural rbs of FlhD (only promoter)
|
| O124
| Oligo-pfliL-Forward-TRO
| TCGAATTCGCGGCCGCTTCTAGAGCAAGGGCGTGTAACAGGCAAC
| 45
|
|
| O125
| Oligo-pfliL-Reverse-TRO
| TCCTGCAGCGGCCGCTACTAGTAGTCATGTGTTGCGGTCTTCCTGTG
| 47
|
|
| O130
| Gene-FlhC-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGATGAGTGAAAAAAGCATTGTTCAGG
| 53
|
|
| O131
| Gene-FlhC-R-TRO
| GTTTCTTCCTGCAGCGGCCGCTACTAGTATTATTAAACAGCCTGTACTCTCTGTTCATCC
| 60
|
|
| O132
| Gene-FlhD-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGATGCATACCTCCGAGTTGCTGAAAC
| 53
| Don't amplify the natural rbs of FlhD
|
| O133
| Gene-FlhD-R-TRO
| GTTTCTTCCTGCAGCGGCCGCTACTAGTATTATTAGGCCCTTTTCTTGCGCAGCGCTTCT
| 60
|
|
| O140
| PlasTest_GFP_Rv
| CCCTGCAGTTAATTAATATAAACGCAGAAAGGCCAC
| 36
| Amplify E0240 with a PacI and a PstI restriction site at the end
|
| O141
| PTst_GFPrbs+_F
| GCCTGCAGTCACACAGGAAAGTACTAGATGC
| 31
| Amplify E0240 with the promoter and a PstI restriction site at the beginning
|
- Preparation of the templates :
Resuspension of 1 colony in 100µl of water.
For each sample,
1 µl dNTP
10 µl Buffer Phusion 5x
2,5 µl Oligo_F
2,5 µl Oligo_R
1µl template
1 µl Phusion
50 µl qsp H2O (33µl)
| Name
| genes
| Oligo
| templates
|
| PCR_130
| Gel E0240 RBS+
| O141_O140
| MG1655
|
| PCR_131
| -
| O141_O140
| Water
|
| PCR_132
| flhD RBS-
| O132_O133
| MG1655
|
| PCR_133
| -
| O132_O133
| Water
|
| PCR_134
| flhC RBS-
| O130_O131
| MG1655
|
| PCR_135
| -
| O130_O131
| Water
|
| PCR_136
| pflhDC
| O110_O131
| MG1655
|
| PCR_137
| -
| O110_O131
| Water
|
| PCR_138
| pflhDC
| O111_O131
| MG1655
|
| PCR_139
| -
| O111_O131
| Water
|
| PCR_140
| pfliL
| O124_O125
| MG1655
|
| PCR_141
| -
| O124_O125
| Water
|
| PCR_142
| pflhDC
| O110_O113
| MG1655
|
| PCR_143
| -
| O110_O113
| Water
|
| PCR_144
| pflhDC
| O111_O113
| MG1655
|
| PCR_145
| -
| O111_O113
| Water
|
- Program PCR_Screening : Annealing 30" at 60°C - Time élongation 1'30" at 72°C - Number cycle : 30
PCR verification/Analysis
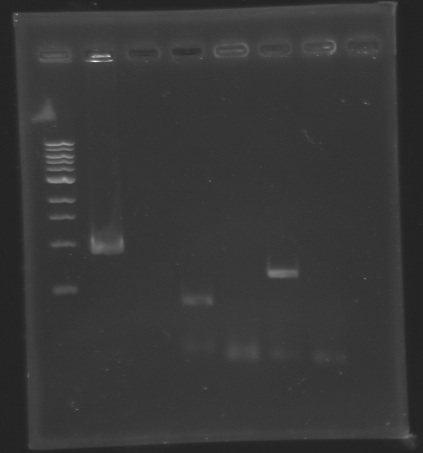 Analysis of PCR product (Gel 1) 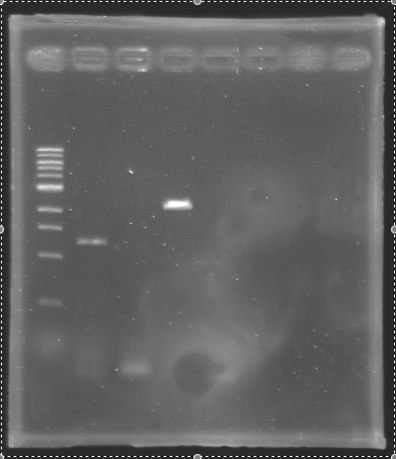 Analysis of PCR product (Gel 2) After the PCR :
- 2*3µl have been analysed by electrophoresis
- the other 44µl of PCR products have been purified by the Promega kit.
ladder : 10µl ladder 1 kb
samples : 3µl of PCR products + 2µl of Loading Dye
migration 30min at 100V, on a 1% agarose gel
| Name
| Promotor
| Gel
| Band
| Expected size
| Measured size
|
| PCR_130
| Gel E0240 RBS+
| Gel 1
| 2
| 876 bp
| 900 bp
|
| PCR_131
| Negative Control
| Gel 1
| 3
| 0 bp
| 0 bp
|
| PCR_132
| flhD RBS-
| Gel 1
| 4
| 351 bp
| 350 bp
|
| PCR_133
| Negative Control
| Gel 1
| 5
| 0 bp
| 0 bp
|
| PCR_134
| flhC RBS-
| Gel 1
| 6
| 579 bp
| 600 bp
|
| PCR_135
| Negative Control
| Gel 1
| 7
| 0 bp
| 0 bp
|
| PCR_136
| pflhDC
| Gel 2
| 2
| 1165 bp
| 1300 bp
|
| PCR_137
| Negative Control
| Gel 2
| 3
| 0 bp
| 0 bp
|
| PCR_138
| pflhDC
| Gel 2
| 4
| 1311 bp
| 2100 pb
|
| PCR_139
| Negative Control
| Gel 2
| 5
| 0 bp
| 0 pb
|
==> Conclusion : We observed the size expected for the PCR products, but not for pflhDC (PCR_138), is right. We hypothesis for PCR_138 that the size is longer that expected due to the aspecific fixation of Oligo O111 (upper to the real site).
ladder : 10µl ladder 100 bp
samples : 3µl of PCR products + 2µl of Loading Dye
migration 30min at 100V, on a 2% agarose gel
| Name
| Promotor
| Gel
| Band
| Expected size
| Measured size
|
| PCR_140
|
| Gel 3
| 2
| 876 bp
| 900 bp
|
| PCR_141
| Negative Control
| Gel 3
| 3
| 0 bp
| 0 bp
|
| PCR_142
|
| Gel 3
| 4
| 351 bp
| 350 bp
|
| PCR_143
| Negative Control
| Gel 3
| 5
| 0 bp
| 0 bp
|
| PCR_144
|
| Gel 3
| 6
| 579 bp
| 600 bp
|
| PCR_145
| Negative Control
| Gel 3
| 7
| 0 bp
| 0 bp
|
Culture of ligation transformants
- 4 clones of each transformation were cultured in 7,5 mL LB + ampicilline. The colonies picked up were the rest of those already picked up from the transformation plate (for the PCR screening of August 8).
- 37°C overnight
| Ligation
| L128
| L129
| L130
|
| Name
| pFlgA
| pFlgB
| pFlhB
|
| Clone N°
| 1
| 2
| 3
| 4
| 1
| 2
| 6
| 7
| 1
| 2
| 7
| 8
|
| Red fluorescence
| yes
| yes
| no
| no
| yes
| yes
| no
| no
| yes
| no
| no
| no
|
|
 "
"


