| Ligation name
| Description
| Antibio
| Nb colonies observed
| Comments
|
| L100
|
rbs TetR - ECFP
D110 (BV) - D130 (BI)
| Amp
| 0
| After another night nothing was osberved -> To do again
|
| L101
|
rbs TetR - GFP tripart
D110 (BV) - D131 (BI)
| Amp
| 1
| After another night nothing was observed -> To do again
|
| L102
|
Strong rbs - YFP
D129 (BV) - D118 (BI)
| Amp
| +/- 100
|
|
| L103
|
Strong rbs - GFP
D129 (BV) - D122 (BI)
| Amp
| a lot
|
|
| L104
|
Strong rbs - lasR activator
D129 (BV) - D114 (BI)
| Amp
| a lot
|
|
| L105
|
Strong promoter - ECFP
D123 (BV) - D130 (BI)
| Amp
| a lot
|
|
| L106
|
Strong promoter - gfp Tripart
D123 (BV) - D131 (BI)
| Amp
| a lot
|
|
| L107
|
Strongest promoter - ECFP
D103 (BV) - D131 (BI)
| Amp
| a lot
|
|
| L108 n°2 (the right one)
|
Strong promoter - gfp Tripart
D103 (BV) - D130 (BI)
| Amp
| +/- 100
|
|
| L109 n°1
|
Strong promoter - ecfp
D124 (BV) - D130 (BI)
| Amp
| +/- 30
| To do again
|
| L109 n°2
|
Strong promoter - ecfp
D124 (BV) - D130 (BI)
| Amp
| a lot
| To do again
|
| L110
|
Medium promoter - gfp Tripart
D124 (BV) - D131 (BI)
| Amp
| a lot
|
|
| L111
|
Weak promoter - ECFP
D104 (BV) - D130 (BI)
| Amp
| a lot
|
|
| L112
|
Weak promoter - gfp
D104 (BV) - D131 (BI)
| Amp
| a lot
|
|
| L113
|
AracpBAD - ecfp
D126 (BV) - D130 (BI)
| Kan
| 1
| After another night nothing was observed -> To do again
|
| L114
|
AracpBAD - gfp tripart
D126 (BV) - D131 (BI)
| Kan
| 2
| After another night nothing was observed -> To do again
|
| L115
|
pLas - ECFP
D105 (BV) - D130 (BI)
| Amp
| a lot
|
|
| L116
|
pLas - gfp Tripart
D105 (BV) - D131 (BI)
| Amp
| a lot
|
|
| L117
|
yfp - Double Terminator
D117 (FI) - D125 (FV)
| Amp
| +/- 30
|
|
| L118
|
rfp - Double Terminator
D121 (FI) - D125 (FV)
| Amp
| +/- 30
|
|
| L119
|
lasR activator + LVA - Double Terminator
D113 (FI) - D125 (FV)
| Amp
| +/- 30
|
|
| L120
|
tetR repressible - ECFP
D106 (BV) - D130 (BI)
| Amp
| a lot
|
|
| L121
|
Strong promoter - gfp tripart
D106 (BV) - D130 (BI)
| Amp
| a lot
|
|
| L122
|
RBS-lasI - ecfp
D107 (BV) - D130 (BI)
| Amp
| 0
| After another night nothing was observed -> To do again
|
| L123
|
RBS lasI - ecfp
D107 (BV) - D131 (BI)
| Amp
| 1
| After another night nothing was observed -> To do again
|
| L124
|
Strongest RBS - rfp
D102 (BV) - D122 (BI)
| Amp
| a lot
|
|
| L125
|
Strongest RBS - yfp
D102 (BV) - D118 (BI)
| Amp
| a lot
|
|
| L126
|
Strongest RBS (1)- LacR activator (+LVA)
D102 (BV) - D118 (BI)
| Amp
| no dish found!!!
| To do again
|
| L127
|
gfp (1)- Double terminator
D119 (FI) - D125 (FV)
| Amp
| no dish found!!!
| To do again
|
| Controls
|
|
|
|
|
| C1
|
D110
|
| 0
| -
|
| C2
|
D129
|
| 5
| -
|
| C3
|
D123
|
| +/- 100
| -
|
| C4
|
D103
|
| +/- 20
| -
|
| C5
|
D124
|
| +/- 100
| -
|
| C6
|
D104
|
| +/- 100
| -
|
| C7
|
D126
|
| 0
| -
|
| C8
|
D105
|
| +/- 20
| -
|
| C9
|
D125
|
| 0
| -
|
| C10
|
D106
|
| 0
| -
|
| C11
|
D107
|
| 0
| -
|
| C12
|
D102
|
| +/- 10
| -
|
| Positive control
|
puc19
|
| 155 (transformation efficiency:1.5*10^7/ug)
| -
|
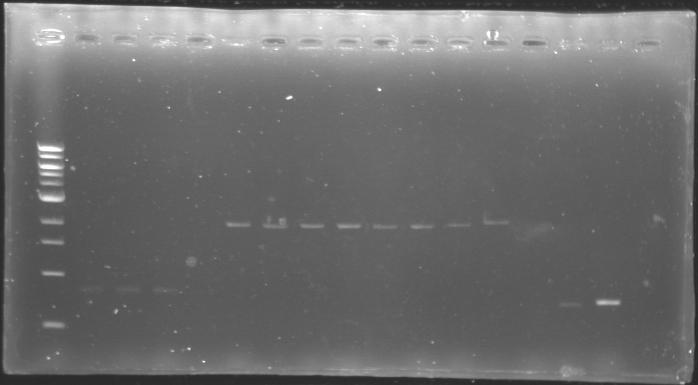
The digested DNA of yesterday was analysed one more time by electrophoresis on a 0.8% agarose gel (about 30 minutes at 100 W). The ladder used was the 1 kb DNA ladder (New England Biolabs). 5 µL of each sample with 1 µL of loading dye were loaded.
 "
"