|
← Yesterday ↓ Calendar ↑Tomorrow →
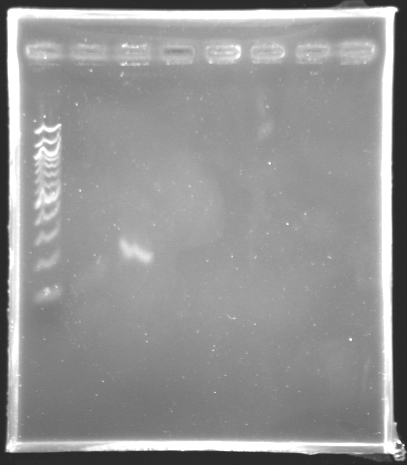
Results of the PCR of the wonderful promoter of fliL
Electrophoresis settings :
- Gel 1.5% agar
- Ladder 100 bp
- Volume of template : 3 µL
| Name
| Promotor
| Well
| Expected size
| Measured size
|
| PCR_135
| pfliL
| 2
| 197 bp
| ~ 200 bp
|
| PCR_135'
| Negative Control
| 3
| 0 bp
| 0 bp
|
Remarks:
We observe that the bands are curved, we suppose that the wells were not very clean.
The size of fliL is good, we will digest it and ligate it today.
Digestion and Ligation of the wonderful promoter of fliL
As the primer used to amplify the promoter of fliL had only two nucleotides after the restriction sites, we tried the two digestions possible : EcoRI + SpeI and XbaI + PstI.
Digestion
Protocol :
| Name
| Genes
| Water
| DNA
| Buffer n°2 10X
| BSA 100X
| Enz 1
| Enz 2
|
| D149
| fliL
| 23 µL
| 2 µl
| 3 µl
| 0.30µl
| EcoRI
| SpeI
|
| D150
| fliL
| 23 µL
| 2 µl
| 3 µl
| 0.30µl
| XbaI
| PstI
|
| D137
| pSB3K3
| 23 µL
| 2 µl
| 3 µl
| 0.30µl
| EcoRI
| SpeI
|
| D152
| pSB3K3
| 23 µL
| 2 µl
| 3 µl
| 0.30µl
| XbaI
| PstI
|
- Incubate 2h30 at 37°C
- 20 min at 65°C to denaturate the enzymes.
Washing of the digestions
We washed the DNA following the standard protocol.
Ligation
- We ligated the DNA following the standard protocol.
- T1 and T2 are the autoligation controls for L143 and L144.
| Ligation name
| Insert name
| Volume of insert µL
| Vector name
| Volume of Vector µL
|
| L 143
| D 149 (pfliL)
| 2
| D 137 (pSB3K3)
| 6
|
| L 144
| D 150 (pfliL)
| 2
| D 152 (pSB3K3)
| 6
|
PCR screening of cloning of E0240, flhD, flhC and pflhDC+flhDC
- PCR screening programm ; elongation time: 1 min 30
- total volume reaction: 50 µL
- primers: O18 & O19
- positive control 1: E0240
- positive control 2: pSB3K3
- negative control: no template
=> Analysis of the results on Tomorrow
|
 "
"