|
← Yesterday ↓ Calendar ↑Tomorrow →
Checking our ligases
Because we couldn't be sure of the results of yesterday experiment, we decided to carry it out one more time.
Digestion
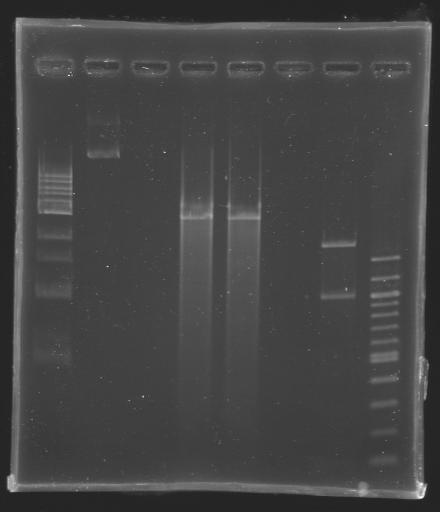
DNA used for digestion: MP101
2 digestion were carried out:
- one with EcoRI (D202), that cuts the plasmid only one time
- the other with BspHI (D203), that cuts the plasmid three times
Reaction mixture
- 10 µL of DNA
- 6µL of 10X buffer 2
- 0,6 µL of 100X BSA
- 2 µL of enzyme (EcoRI or BspHI)
- 41,4 µL of water
2h30 at 37°C and then 20 min at 65°C
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
|
| sample
| 1 kb ladder
| MP101
1 µL
|
| D202
10 µL
| D202
10 µL
|
| D203
3 µL
| 100 bp ladder
|
| expected size
|
|
|
| 2984 bp
|
| 1870 bp
1008 bp
105 bp
|
|
| measured size
|
|
|
| 3 kb
| 3 kb
|
| 1,9 kb
1 kb
|
|
Purification
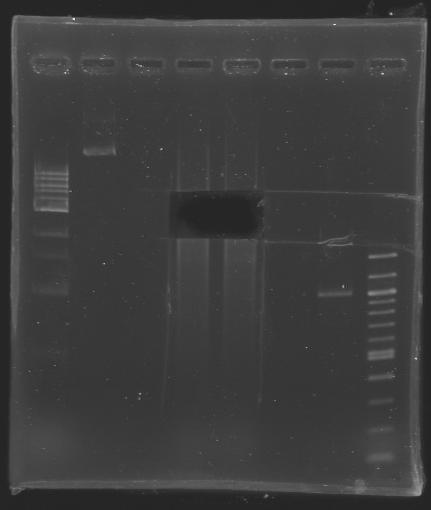
 After purification well n°2: D202 purified by gel well n°3: D202 purified by column well n°4: D203 purified by column well n°5: MP101 D202 (EcoRI digestion) was purified in 2 ways:
- by gel extraction
- or by column
D203 (BspHI digestion) was purified:
Elution in 30 µL of EB buffer
Ligation
3 ligases tested
- ligase 1: our 250 µL (400 000 U/mL) tube
- ligase 2: our 50 µL (400 000 U/mL) tube
- ligase 3: 2nd floor 50 µL (400 000 U/mL) tube
For the samples for which the digestion products have not been purified, 1 µL of ligase and 1 µL of ATP (1 mM final concentration) have been added directly in the digestion products (in the digestion buffer 2).
All the ligation were carried out overnight at 4°C.
| ligation
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
| 9
| 10
| 11
| 12
| 13
|
| digested DNA used
| D202 (EcoRI digestion)
| D203 (BspHI digestion)
|
type of purification
of the digestion products
| gel extraction
| column
| no
purification
| column
| no
purification
|
| ligase used
| L1
| L2
| L3
| L1
| L2
| L3
| L1
| L1
| L2
| L3
| L1
| L2
| L3
|
|
 "
"