|
← Yesterday ↓ Calendar ↑Tomorrow →
Construction for Synchronization
Transformation of the ligations we did yesterday
| Ligation Name
| Description
| Antibio
|
| L159
| D125 (FV) + D109 (FI)
| Kan
|
| Control L159
| D125
| Kan
|
| L162
| D107 (BV) + D163 (BI)
| Amp
|
| Control L162
| D107
| Amp
|
| L163
| D110 (BV) + D163 (BI)
| Amp
|
| Control L163
| D110
| Amp
|
Construction of pFlgA - GFP Generator
Aim : Construction of "pFlgA-RBS-GFP-dbl ter" (pFlgA-E0240)     
| Ligation name
| Description
| Antibio
| Number Colonies observed
| Fluorescence
| Comments
|
| Ligations
|
| L164
| D168(FV) - D132(FI)
pFlgA - gfp generator
| Amp
| 125
| No
| ok
|
| Controls
|
| TL164
| D168(FV)
| Amp
| 8
| No
| ok
|
| Positive Control
| pUC19
| Amp
| 2000 (efficiency 2.10^8)
| No
| OK
|
=> Need to screen to know which clones we can use for the of pFlgA promotor characterization.
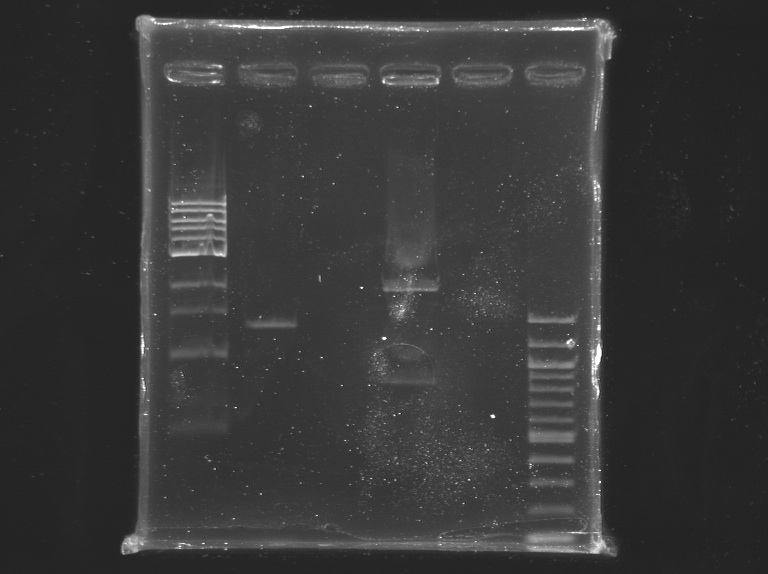
Cloning of EnvZ*
The sequencing of EnvZ* previously cloned, revealed a loss of about 300 bp. EnvZ* contains indeed an EcoRI restriction site within its sequence. So we can't use this enzyme during the cloning.
Digestion
| digestion number
| name
| template
| Enzymes
|
| D159
| EnvZ*
| PCR129 from August 8th
| XbaI & PstI
|
| D116
| pSB1A2
| MP108 (C0179 (lasR-pSB1A2))
| XbaI & PstI
|
Reaction mixture
- 4 µL of PCR129 (or 2 µL of MP108)
- 3 µL of 10X buffer n°2
- 0,3 µL of 100X BSA
- 1 µL of XbaI
- 1 µL of PstI
- 20,7 µL (or 22,7 µL) of water
Incubation at 37°C during 2H25, and then ~20 min at 65°C
Electrophoresis
1% agarose gel
- EnvZ*: 3 µL of digestion products + 1 µL of loading blue + 2 µL of water
- pSB1A2: 30 µL of digestion products + 6 µL of loading blue
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
|
| sample
| 1 kb DNA ladder
| D159 (EnvZ*)
| nothing
| D116 (pSB1A2 & lasR)
| nothing
| 100 bp DNA ladder
|
| expected size
|
| 1421 bp
|
| 2057 bp & 707 bp
|
|
|
| measured size
|
| 1,4 kb
|
| 2 kb & 0.7 kb
|
|
|
Purification
- EnvZ*: digestion product purified directly by Qiagen kit
- pSB1A2: excision of the band (2 kb) from the gel and purification by the Qiaquick Gel Extraction kit
elution in 30 µL of buffer EB
then kept at - 20°C
Screening of the cloning of pFlgA-YFP Tripart (LVA+/-)
Electrophoresis
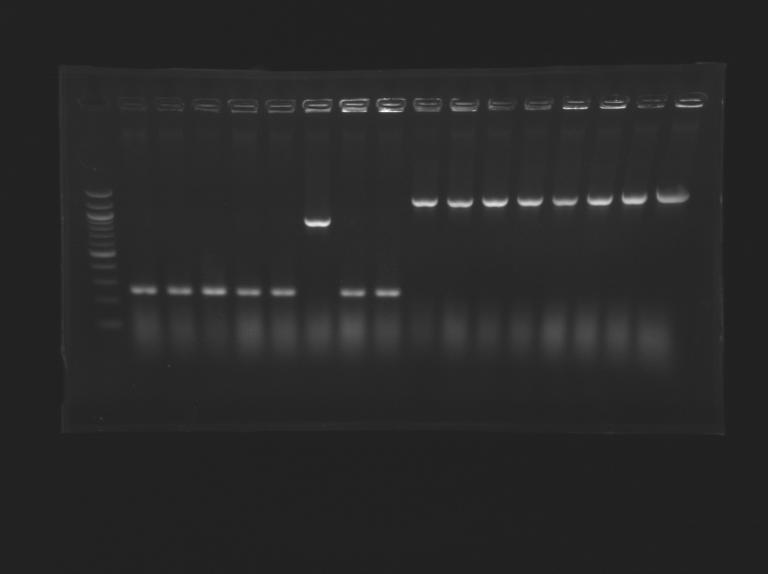 Gel n°1 : Screening of L160 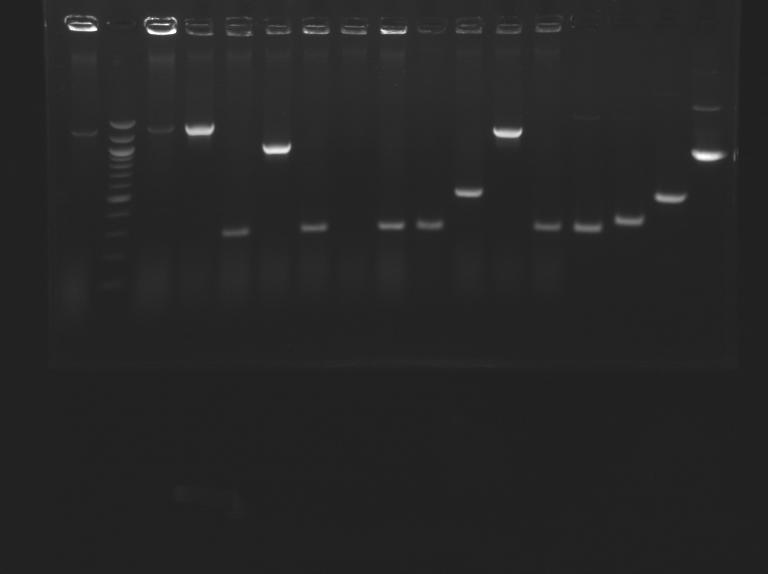 Gel n°2 : Screening of L161
|
| Gel n° 1
|
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
| 9
| 10
| 11
| 12
| 13
| 14
| 15
| 16
|
| sample
| 100pb ladder
| don't matter
| L160.1
| L160.2
| L160.3
| L160.4
| L160.5
| L160.6
| L160.7
| L160.8
|
| expected size (pb)
|
|
| 1 200
|
| measured size (pb)
|
|
| 1 200
| 1 200
| 1 200
| 1 200
| 1 200
| 1 200
| 1 200
| 1 200
|
|
| Gel n° 2
|
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
| 9
| 10
| 11
| 12
| 13
| 14
| 15
| 16
|
| sample
| L161.1
| 100 pb ladder
| L161.2
| L161.3
| don't matter
|
| expected size (pb)
| 1 167
|
| 1 167
|
| measured size (pb)
| 1 300
|
| 1 300
| 1 30
|
Minipreps and glycerol stock
| Miniprep
| Glycerol Stock
| Ligation
| Name
|
| MP1
| S1
| L160
| FlgA-rbs-YFP-dbl ter
|
| MP1
| S1
| L161
| FlgA-rbs-YFP-LVA+-dbl ter
|
Promoter characterization plasmids
PCR screenning: transformation results from August 23th
Protocol
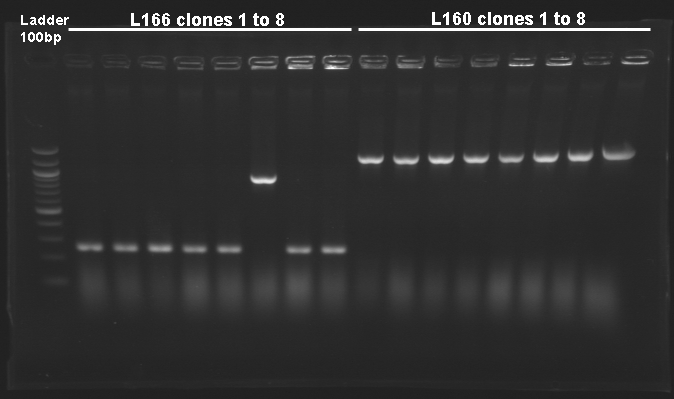
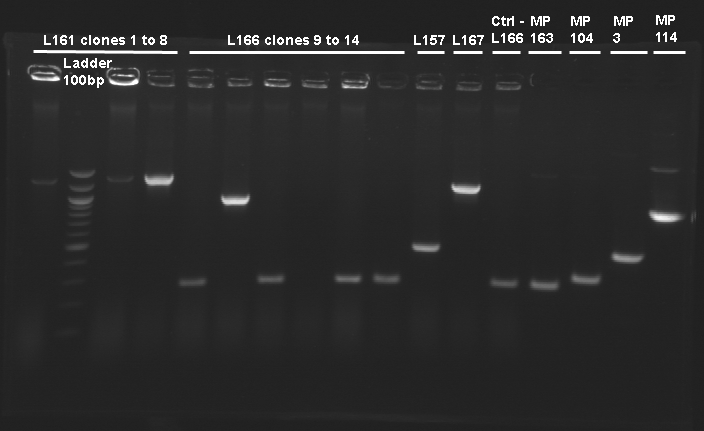
| Ligation name
| Clone number
| Product description
| Primers used
| Size expected
| Size observed
|
| no name ligation
| 1
| tetR-B0015
| O18-O19
|
|
|
| Control L166
| 1
| Vector autoligation control
| O18-O19
| 249
| correct
|
| L166
| 1-5,7-9,11-14
| RBS B0032 - tetR
| O18-O19
|
| around 249
|
| L166
| 6
| RBS B0032 - tetR
| O18-O19
|
|
|
| L166
| 10
| RBS B0032 - tetR
| O18-O19
|
|
|
| L167
| 1
| gfp generator - pTet
| O18-O19
|
|
|
|
 "
"