Team:Paris/August 12
From 2008.igem.org
(Difference between revisions)
(→Glycerol Stocks) |
(→Minipreps: Plasmid extraction) |
||
| Line 2: | Line 2: | ||
| + | ==Digestion of the PCR we did yesterday== | ||
| + | Yesterday we amplified ''flhD'', ''flhC'', ''flhDC'' with its promoter and ''RBS+'' | ||
| + | Before the digestion, we have to determine the DNA concentration of the templates. | ||
| + | |||
| + | ==='''Measurement of DNA concentration'''=== | ||
| + | We used the biophotometer. We put 5 µL of template DNA in 95 µL of water. The Blank was made with 5 µL of EB Buffer (the one that was used for the elution of the DNA) in 95 µL of water. | ||
| + | {| Border="2" | ||
| + | |align="center"|'''Template''' | ||
| + | |align="center"|'''Concentration '''<br>(µg/µL) | ||
| + | |- | ||
| + | |align="center"| PCR 130 <br> E0240 RBS + | ||
| + | |align="center"| 0.44 | ||
| + | |- | ||
| + | |align="center"| PCR 131 <br> ''flhD'' RBS - | ||
| + | |align="center"| 0.06 | ||
| + | |- | ||
| + | |align="center"| PCR 132 <br> ''flhC'' RBS - | ||
| + | |align="center"| 0.12 | ||
| + | |- | ||
| + | |align="center"| PCR 133 <br> ''flhDC'' with promoter | ||
| + | |align="center"| 0.08 | ||
| + | |- | ||
| + | |align="center"| MP 142 <br> pSB3K3 | ||
| + | |align="center"| 0.04 | ||
| + | |- | ||
| + | |align="center"| MP 122 <br> pSB1A2 | ||
| + | |align="center"| 0.2 | ||
| + | |- | ||
| + | |} | ||
| + | ==='''Digestion'''=== | ||
| + | ====Protocol==== | ||
| + | {| Border="2" | ||
| + | |align="center"|'''Digestion name''' | ||
| + | |align="center"|'''Template DNA''' | ||
| + | |align="center"|''' Enzymes ''' | ||
| + | |align="center"|'''Volume of DNA''' | ||
| + | |- | ||
| + | |align="center"|D 137 | ||
| + | |align="center"| | ||
| + | |align="center"| PstI | ||
| + | |align="center"|25 µL | ||
| + | |- | ||
| + | |align="center"|D 138 | ||
| + | |align="center"|MP 143 - E0240 | ||
| + | |align="center"|EcoRI-SpeI | ||
| + | |align="center"|6.25 µL | ||
| + | |} | ||
| + | |||
| + | * X µL of Template DNA | ||
| + | * Buffer (n°2) 10X : 3µL | ||
| + | * BSA 100X : 0.3µL | ||
| + | * Pure water qsp 30 µL | ||
| + | * 1 µL of each enzyme | ||
| + | |||
| + | * Incubate during about 3h at 37°C, then 20 minutes at 65°C (to inactivate the enzymes). | ||
| + | |||
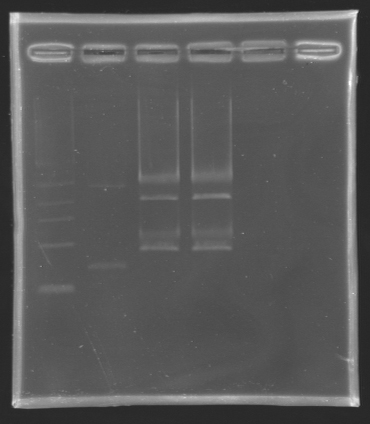
| + | ====Results of the digestion==== | ||
| + | |||
| + | [[Image:KR000131.jpg|thumb|Result of the digestion to build the measurement plasmid]] | ||
| + | |||
| + | '''Electrophoresis settings''' | ||
| + | * Gel : 1 % agar | ||
| + | * 4µL template DNA for D137 | ||
| + | * All the digestion product for D138 because we have to separate two products, the backbone measures 2056 bp and the insert we want to extract is 899 bp long. | ||
| + | * 10µL QuickLoad DNA ladder 1 kb | ||
| + | |||
| + | |||
| + | {| border="1" | ||
| + | |align="center"|'''Name''' | ||
| + | |align="center"|'''Band''' | ||
| + | |align="center"|'''Expected size''' | ||
| + | |align="center"|'''Measured size''' | ||
| + | |- | ||
| + | |align="center"|D 137 | ||
| + | |align="center"|2 | ||
| + | |style="background: #cbff7B"|<center>2727 bp</center> | ||
| + | |align="center"|3000 bp | ||
| + | |- | ||
| + | |align="center"|D 138 | ||
| + | |align="center"|3&4 | ||
| + | |style="background: #cbff7B"|<center>899 bp</center> | ||
| + | |align="center"|~1000 bp | ||
| + | |} | ||
| + | |||
| + | ==='''Gel extraction and DNA purification'''=== | ||
| + | To extract the biobrick E0240 from the gel, we used the standard protocol [[Team:Paris/Notebook/Protocols#Extraction|number 8]].<br> | ||
| + | To purify the DNA we used the standard protocol [[Team:Paris/Notebook/Protocols#Purification_.28Kit_Promega.29|number 10]] | ||
| + | |||
| + | The ligation will be done [[Team:Paris/August_9|tomorrow]]. | ||
== Minipreps: Plasmid extraction== | == Minipreps: Plasmid extraction== | ||
Revision as of 14:03, 14 August 2008
|
Digestion of the PCR we did yesterdayYesterday we amplified flhD, flhC, flhDC with its promoter and RBS+ Before the digestion, we have to determine the DNA concentration of the templates. Measurement of DNA concentrationWe used the biophotometer. We put 5 µL of template DNA in 95 µL of water. The Blank was made with 5 µL of EB Buffer (the one that was used for the elution of the DNA) in 95 µL of water.
DigestionProtocol
Results of the digestionElectrophoresis settings
Gel extraction and DNA purificationTo extract the biobrick E0240 from the gel, we used the standard protocol number 8. The ligation will be done tomorrow. Minipreps: Plasmid extraction
Glycerol Stocks
Results of the transformations we did yesterdayYour results please Cyprien!!!??? Concentration of the MiniPreps
New PCR screening with the right primersTransformants with pFlgA, pFlgB and pFlhB cloned into J61002 are analysed by PCR but this time with the right primers: VF2 (O18) and VR (O19).
Electrophoresis
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"