Team:Paris/August 21
From 2008.igem.org
(Difference between revisions)
(→Gel Extraction) |
AnaJimenez (Talk | contribs) (→Ligations) |
||
| Line 393: | Line 393: | ||
|D109 (FI) | |D109 (FI) | ||
|1.15 | |1.15 | ||
| + | |} | ||
| + | |||
| + | |||
| + | =Promoter characterization plasmids= | ||
| + | |||
| + | ==Ligation== | ||
| + | |||
| + | Our ligations from yesterday didn't work. The positive control for transformation worked. | ||
| + | |||
| + | ==Digestion== | ||
| + | |||
| + | We had a problem with a gel extraction so we have to make again the digestions from yesterday | ||
| + | |||
| + | |||
| + | Other digestions made: | ||
| + | |||
| + | |||
| + | |||
| + | [[Team:Paris/Notebook/Protocols#Digestion|Protocol Digestion]] | ||
| + | |||
| + | {| border="1" style="text-align: center" | ||
| + | |'''Digestion name''' | ||
| + | |'''Plasmid''' | ||
| + | |'''Description''' | ||
| + | |'''Miniprep used''' | ||
| + | |'''Enzymes''' | ||
| + | |'''Concentration after gel extraction''' | ||
| + | |- | ||
| + | |D179 | ||
| + | |MP3.4 | ||
| + | |B0015 (double terminator B0010-B0012) - BV | ||
| + | |4 | ||
| + | |SpeI and PstI | ||
| + | |9 | ||
| + | |- | ||
| + | |D180 | ||
| + | |MP101.1 | ||
| + | |promoter J23101- BV | ||
| + | |1 | ||
| + | |SpeI and PstI | ||
| + | |7 | ||
| + | |- | ||
| + | |D181 | ||
| + | |MP104.2 | ||
| + | |PTet (TetR repressible promoter) - FV | ||
| + | |1 | ||
| + | |EcoRI and XbaI | ||
| + | |1 | ||
| + | |- | ||
| + | |D182 | ||
| + | |MP114.1 | ||
| + | |TetR - BI | ||
| + | |1 | ||
| + | |XbaI and PstI | ||
| + | |10 | ||
| + | |- | ||
| + | |D183 | ||
| + | |MP119.3 | ||
| + | |pBad promoter - BI | ||
| + | |1 | ||
| + | |XbaI and PstI | ||
| + | |0 | ||
| + | |- | ||
| + | |D184 | ||
| + | |MP143.1 | ||
| + | |gfp generator - FI | ||
| + | |2 | ||
| + | |EcoRI and SpeI | ||
| + | |13 | ||
| + | |- | ||
| + | |D185 | ||
| + | |MP163.1 | ||
| + | |B0032 RBS - BV | ||
| + | |2 | ||
| + | |SpeI and PstI | ||
| + | |21 | ||
| + | |} | ||
| + | |||
| + | |||
| + | D179 | ||
| + | D180 | ||
| + | D181 | ||
| + | D182 | ||
| + | D183 | ||
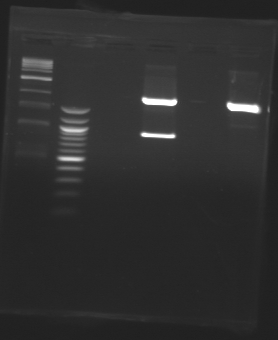
| + | [[Image:20-08-08.png|200px]] | ||
| + | [[Image:20-08-08-coupe.png|200px]] | ||
| + | |||
| + | D184 | ||
| + | D185 | ||
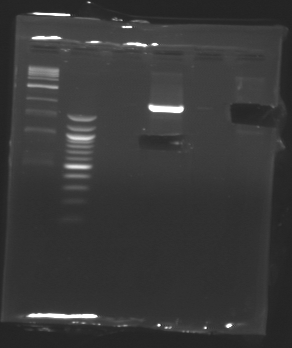
| + | [[Image:20-08-08bis.png|100px]] | ||
| + | [[Image:20-08-08bis-coupe.png|100px]] | ||
| + | |||
| + | ==Ligation== | ||
| + | |||
| + | |||
| + | [[Team:Paris/Notebook/Protocols#Ligation |Protocol]] | ||
| + | |||
| + | |||
| + | {|border="1" style="text-align: center" | ||
| + | |'''Ligation name''' | ||
| + | |'''Vector digestion''' | ||
| + | |'''Vector description''' | ||
| + | |'''Vector volume''' | ||
| + | |'''Insert digestion''' | ||
| + | |'''Insert description''' | ||
| + | |'''Insert volume''' | ||
| + | |'''Product description''' | ||
| + | |'''Antibiotic''' | ||
| + | |- | ||
| + | |L155 | ||
| + | |D164 | ||
| + | |J23101 promoter | ||
| + | |10 | ||
| + | |D163 | ||
| + | |gfp generator | ||
| + | |2 | ||
| + | |J23101 promoter-gfp generator | ||
| + | |Amp | ||
| + | |- | ||
| + | |L156 | ||
| + | |D161 | ||
| + | |pTet promoter | ||
| + | |1 | ||
| + | |D163 | ||
| + | |gfp generator | ||
| + | |4 | ||
| + | |pTet promoter-gfp generator | ||
| + | |Kana | ||
| + | |- | ||
| + | | | ||
| + | |D161 | ||
| + | | | ||
| + | |1 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Vector autoligation control | ||
| + | |Kana | ||
| + | |- | ||
| + | |L157 | ||
| + | |D125.2 | ||
| + | |B0015 | ||
| + | |3 | ||
| + | |D162 | ||
| + | |4 | ||
| + | |tetR | ||
| + | |tetR-B0015 | ||
| + | |Amp | ||
| + | |- | ||
| + | | | ||
| + | |D125.2 | ||
| + | | | ||
| + | |3 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Vector autoligation control | ||
| + | |Amp | ||
|} | |} | ||
Revision as of 17:26, 25 August 2008
Cloning of FlhB promoterProtocol
Resuspension of 1 colony E.coli K12 strain MG 1655 in 100µl of water.
1µl of dNTP
Result[[Image:KR00019b.jpg| thumb| Vérification of pFlhB]
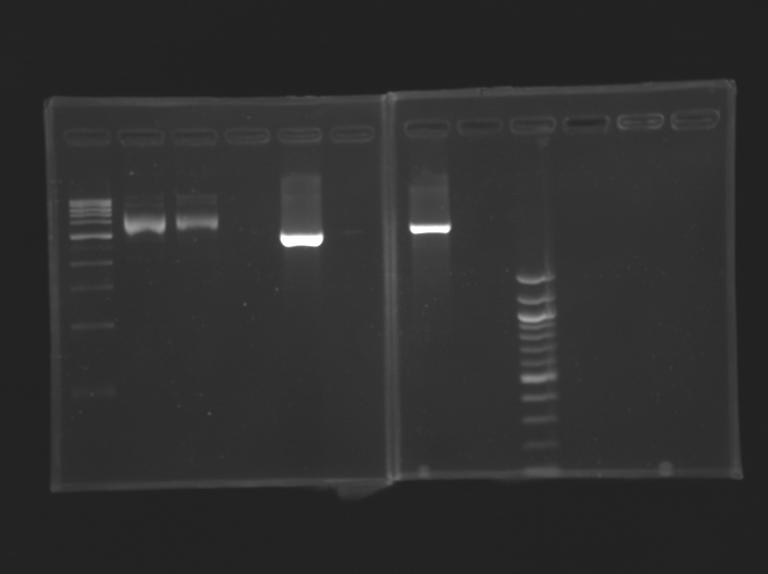
Construction for FIFOAim : Construction of pFlgA - YFP tripart (+/- LVA) "pFlgA-RBS-YFP-dbl ter" (pFlgA-E0430/E0432) DigestionDigestion
Gel Extraction
Measurement of the concentration of D166 & D167 purifiedProtocol (it's same that for Miniprep)
Ligation
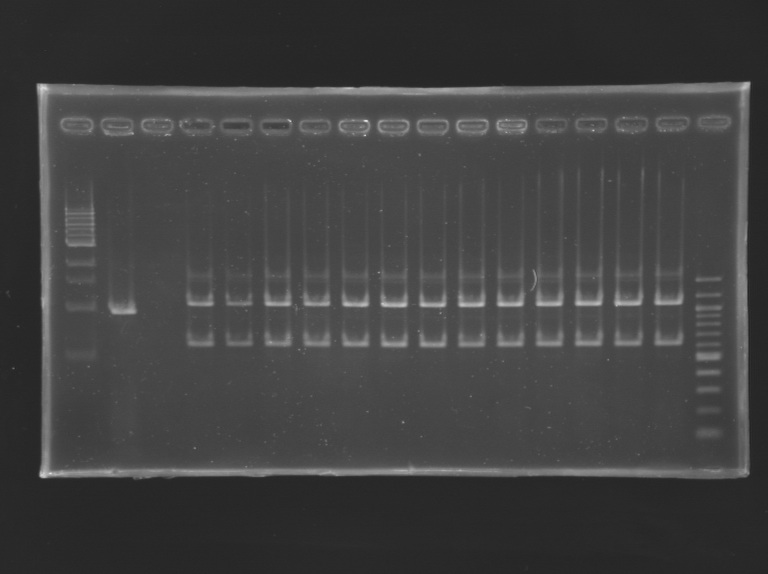
Screening of the cloning of E0240 and FlhDC+promotorWe obtained single colonies with the dilution 100.
PCR screeningreaction mixture (25 µL)
PCR screening programm
Electrophoresis
Construction for synchronizationLigations
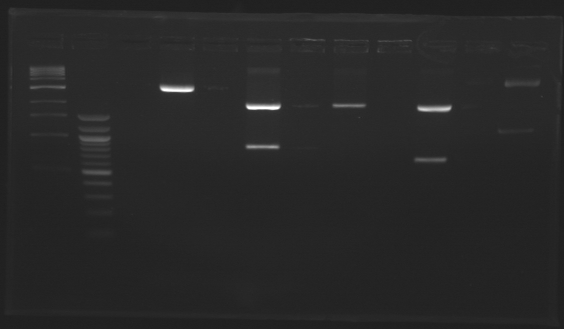
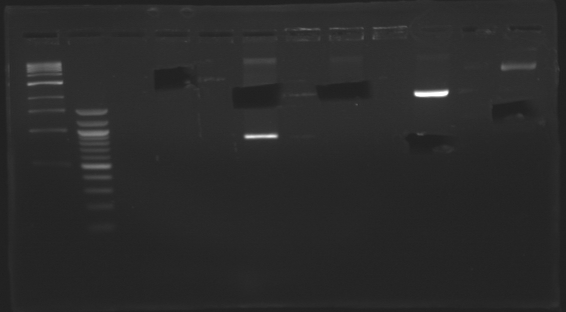
Promoter characterization plasmidsLigationOur ligations from yesterday didn't work. The positive control for transformation worked. DigestionWe had a problem with a gel extraction so we have to make again the digestions from yesterday
Ligation
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"