Team:Paris/August 22
From 2008.igem.org
(Difference between revisions)
(→Transformation of the ligations we did yesterday) |
(→Transformation of the ligations we did yesterday) |
||
| (32 intermediate revisions not shown) | |||
| Line 73: | Line 73: | ||
|'''measured size''' | |'''measured size''' | ||
| | | | ||
| - | |'''1 kb'''<br>3 kb | + | |style="background: #ff6d73" |'''1 kb'''<br>3 kb |
| | | | ||
| - | |0,3 kb<br>1,8 kb | + | |style="background: #ff6d73" |0,3 kb<br>1,8 kb |
| - | |0,3 kb<br>1,8 kb | + | |style="background: #cbff7B"| 0,3 kb<br>1,8 kb |
| - | |0,9 kb<br>2 kb | + | |style="background: #ff6d73" |0,9 kb<br>2 kb |
| - | |2 kb | + | |style="background: #ff6d73" |2 kb |
| - | |2 kb | + | |style="background: #ff6d73" |2 kb |
| | | | ||
|} | |} | ||
| - | + | ||
| + | '''Results''': The clones tested didn't have the insert. | ||
| + | |||
='''Construction of pFlgA - GFP Generator'''= | ='''Construction of pFlgA - GFP Generator'''= | ||
| - | Aim : Construction of ''' "pFlgA-RBS-GFP-dbl ter" (pFlgA- | + | Aim : Construction of ''' "pFlgA-RBS-GFP-dbl ter" (pFlgA-E0240)''' [[Image:Part_icon_regulatory.png]][[Image:Part_icon_rbs.png]][[Image:icon_reporter.png]][[Image:Part_icon_terminator.png]][[Image:Part_icon_terminator.png]] |
==Digestion== | ==Digestion== | ||
| Line 111: | Line 113: | ||
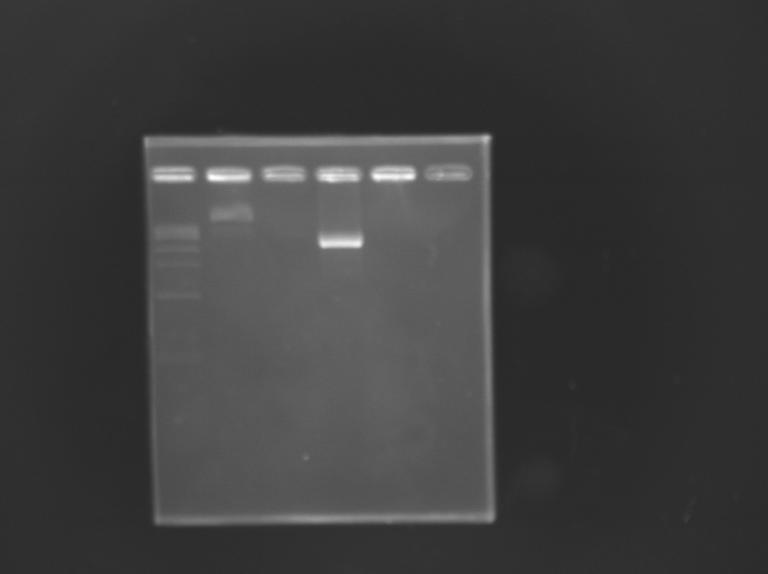
==='''Gel Extraction'''=== | ==='''Gel Extraction'''=== | ||
[[Team:Paris/Notebook/Protocols#Migration after extraction |Protocol]] | [[Team:Paris/Notebook/Protocols#Migration after extraction |Protocol]] | ||
| - | [[Image: | + | [[Image:KR000222b.JPG |thumb |Gel Extraction of D168]] |
{|border="1" style="text-align: center" | {|border="1" style="text-align: center" | ||
| Line 133: | Line 135: | ||
| | | | ||
| | | | ||
| - | | | + | |2 940 |
| + | | colspan="2"| | ||
|- | |- | ||
|'''Measured size (pb)''' | |'''Measured size (pb)''' | ||
| | | | ||
| - | |style="background: #cbff7B"| | + | |style="background: #cbff7B"|5.000 |
| - | + | ||
| - | + | ||
| | | | ||
| + | |style="background: #cbff7B"|3 000 | ||
| + | |colspan="2"| | ||
|} | |} | ||
| Line 153: | Line 156: | ||
|- | |- | ||
|D168 | |D168 | ||
| - | | | + | |24 |
| - | | | + | |2.15 |
|} | |} | ||
| Line 169: | Line 172: | ||
|L164 | |L164 | ||
|D168 | |D168 | ||
| - | | | + | |1.67 |
|D132 | |D132 | ||
| - | | | + | |2.04 |
|- | |- | ||
|Control L164 | |Control L164 | ||
|D168 | |D168 | ||
| - | | | + | |1.67 |
| - | | - | ||
| - | | - | ||
|} | |} | ||
| + | |||
='''Construction for FIFO'''= | ='''Construction for FIFO'''= | ||
| Line 193: | Line 197: | ||
|- | |- | ||
|L160 | |L160 | ||
| - | |D166 (FV) + D132 (FI) | + | |D166 (FV) + D132 (FI)<br>pFlgA - YFP tripart (LVA-) |
|Amp | |Amp | ||
|- | |- | ||
| - | | | + | |TL160 |
| - | |D166 | + | |D166<br> YFP tripart (LVA-) |
|Amp | |Amp | ||
|- | |- | ||
|L161 | |L161 | ||
| - | |D167 (FV) + | + | |D167 (FV) + D132 (FI)<br>pFlgA - YFP tripart (LVA+) |
|Amp | |Amp | ||
|- | |- | ||
| - | | | + | |TL161 |
| - | |D167 | + | |D167<br>YFP tripart (LVA+) |
|Amp | |Amp | ||
|} | |} | ||
| Line 227: | Line 231: | ||
|- | |- | ||
|L159 | |L159 | ||
| - | |D125 (FV)+D109(FI) | + | |D125 (FV) + D109 (FI) |
|Kan | |Kan | ||
|- | |- | ||
| Line 284: | Line 288: | ||
|- | |- | ||
|L162 | |L162 | ||
| - | |D107(BV) + D163 (BI) | + | |D107 (BV) + D163 (BI) |
|Amp | |Amp | ||
|- | |- | ||
| Line 292: | Line 296: | ||
|- | |- | ||
|L163 | |L163 | ||
| - | |D110(BV)+D163(BI) | + | |D110 (BV) + D163 (BI) |
|Amp | |Amp | ||
|- | |- | ||
|Control L163 | |Control L163 | ||
|D110 | |D110 | ||
| + | |Amp | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | ='''Promoter characterization plasmids'''= | ||
| + | |||
| + | ==Transformation of ligations from August 20th== | ||
| + | [[Team:Paris/Notebook/Protocols#Transformation |Protocol]] | ||
| + | |||
| + | '''Top 10 cells were used''' | ||
| + | |||
| + | {|border="1" style="text-align: center" | ||
| + | |'''Ligation name''' | ||
| + | |'''Vector digestion''' | ||
| + | |'''Vector description''' | ||
| + | |'''C° Vector (µg/mL)''' | ||
| + | |'''V. Vector (µl)''' | ||
| + | |'''Insert digestion''' | ||
| + | |'''Insert description''' | ||
| + | |'''C° Insert (µg/mL)''' | ||
| + | |'''V. Insert (µl)''' | ||
| + | |'''Product description''' | ||
| + | |'''Antibiotic''' | ||
| + | |- | ||
| + | |L155 | ||
| + | |D164 | ||
| + | |J23101 promoter | ||
| + | |3 | ||
| + | |16 | ||
| + | |D163 | ||
| + | |gfp generator | ||
| + | |14 | ||
| + | |8 | ||
| + | |J23101 promoter-gfp generator | ||
| + | |Amp | ||
| + | |- | ||
| + | |L156 | ||
| + | |D161 | ||
| + | |pTet promoter | ||
| + | |39 | ||
| + | |1.25 | ||
| + | |D163 | ||
| + | |gfp generator | ||
| + | |14 | ||
| + | |5 | ||
| + | |pTet promoter-gfp generator | ||
| + | |Kana | ||
| + | |- | ||
| + | |Control L156 | ||
| + | |D161 | ||
| + | | | ||
| + | | | ||
| + | |1.25 | ||
| + | |none | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Vector autoligation control | ||
| + | |Kana | ||
| + | |- | ||
| + | |L157 | ||
| + | |D125.2 | ||
| + | |B0015 | ||
| + | |15 | ||
| + | |3 | ||
| + | |D162 | ||
| + | |tetR | ||
| + | |11 | ||
| + | |4 | ||
| + | |tetR-B0015 | ||
| + | |Kana | ||
| + | |- | ||
| + | |Control L157 | ||
| + | |D125.2 | ||
| + | | | ||
| + | | | ||
| + | |3 | ||
| + | |none | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Vector autoligation control | ||
| + | |Kana | ||
| + | |- | ||
| + | |L166 | ||
| + | |D185 | ||
| + | |RBS B0032 | ||
| + | |21 | ||
| + | |2 | ||
| + | |D182 | ||
| + | |tetR | ||
| + | |10 | ||
| + | |6.5 | ||
| + | |RBS B0032 - tetR | ||
| + | |Amp | ||
| + | |- | ||
| + | |Control L166 | ||
| + | |D185 | ||
| + | | | ||
| + | | | ||
| + | |2 | ||
| + | |none | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Vector autoligation control | ||
| + | |Amp | ||
| + | |- | ||
| + | |L167 | ||
| + | |D181 | ||
| + | |pTet | ||
| + | |1 | ||
| + | |14 | ||
| + | |D184 | ||
| + | |gfp generator | ||
| + | |14 | ||
| + | |3 | ||
| + | |gfp generator - pTet | ||
| + | |Amp | ||
| + | |- | ||
| + | |Control L167 | ||
| + | |D181 | ||
| + | |pTet | ||
| + | | | ||
| + | |14 | ||
| + | |none | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Vector autoligation control | ||
|Amp | |Amp | ||
|} | |} | ||
Latest revision as of 00:54, 21 September 2008
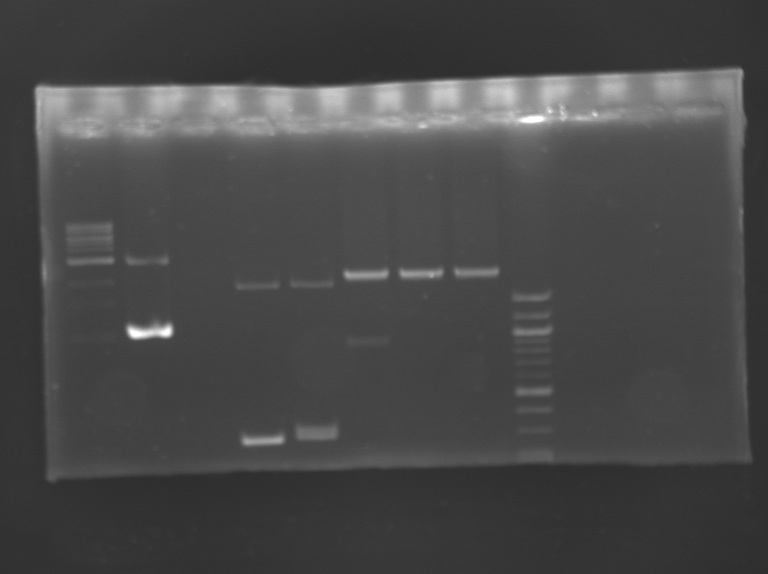
Analysis of the transformant of FlhDC+promotor
PCRPCR screening programm
Digestiontotal volume reaction (30 µL)
Incubation 2h55 at 37°C and then 20 min at 65°C. Electrophoresis
Results: The clones tested didn't have the insert.
Construction of pFlgA - GFP GeneratorAim : Construction of "pFlgA-RBS-GFP-dbl ter" (pFlgA-E0240) DigestionDigestion
Gel Extraction
Measurement of the concentration of D168 purifiedProtocol (it's same that for Miniprep)
Ligation
Construction for FIFOAim : Construction of pFlgA - YFP tripart (+/- LVA) "pFlgA-RBS-YFP-dbl ter" (pFlgA-E0430/E0432) Transformation of the ligations we did yesterday
Construction for synchronizationTransformation of the ligations we did yesterday
LigationTransformation
Promoter characterization plasmidsTransformation of ligations from August 20thTop 10 cells were used
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"