Team:Paris/July 26
From 2008.igem.org
(Difference between revisions)
(→Results of digestions : Electrophoresis) |
(→Extraction of the DNA) |
||
| (One intermediate revision not shown) | |||
| Line 114: | Line 114: | ||
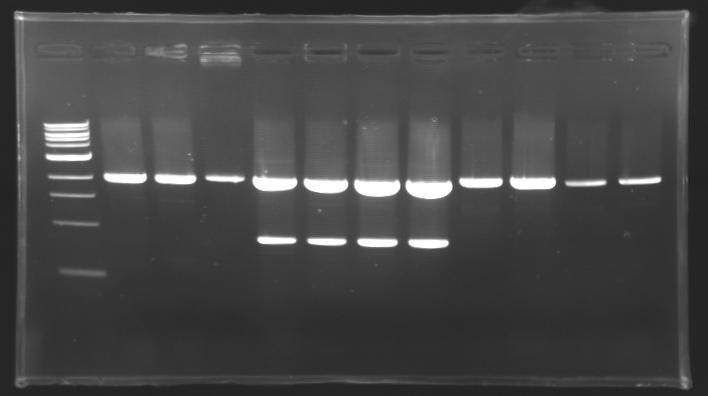
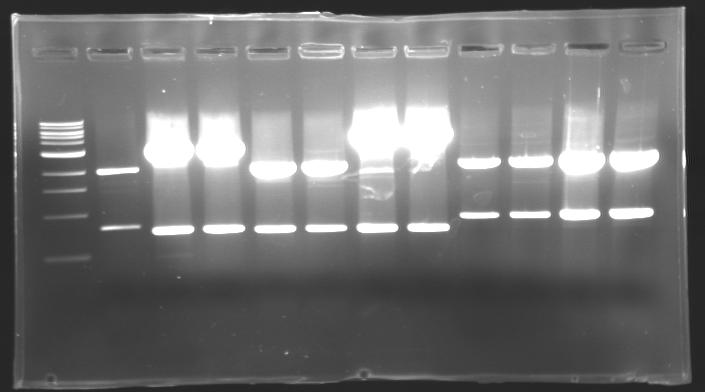
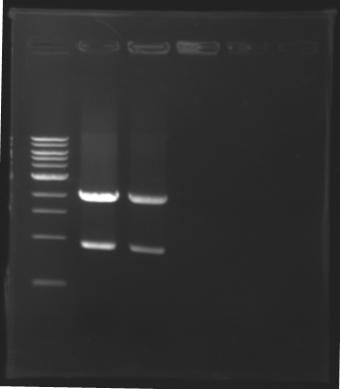
| - | + | === Results of digestions : Electrophoresis === | |
| - | conditions : | + | '''conditions :''' |
| + | |||
* 10µl of ladder 1 kb (except for gel n°6 : 100 pb) | * 10µl of ladder 1 kb (except for gel n°6 : 100 pb) | ||
* 30µl of digestion added with 5µl of loading Dye 6x | * 30µl of digestion added with 5µl of loading Dye 6x | ||
| Line 130: | Line 131: | ||
| - | gel5 [[Image: | + | gel5 [[Image:080726gel_5.jpg| gel5|100px]] |
| - | gel6 [[Image: | + | gel6 [[Image:080726gel_6.jpg| gel6|100px]] |
| Line 643: | Line 644: | ||
==> conclusion : all the digestion have succeed.....GREAT ! | ==> conclusion : all the digestion have succeed.....GREAT ! | ||
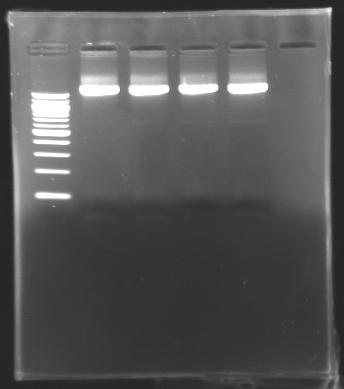
| - | == | + | == DNA extraction == |
Latest revision as of 16:55, 31 July 2008
|
MiniPreps
DigestionDigestion Mix10µl of Miniprep (26 aug.)
Results of digestions : Electrophoresisconditions :
DNA extraction
|
 "
"